Benzene & Benzene Derivatives Available For Purchase
At Chempanda, we offer a range of benzene and benzene derivative chemical compounds that can be shipped to anywhere across the globe. We offer over 37960 different compounds that are available in varying purities and quantities.
Our Benzene Suppliers
We have partnered with a range of chemical manufacturers who specialize exclusively in synthesizing benzene and their derivatives. With years of industry experience, our suppliers offer the most common benzene compounds, or can custom manufacture benzene compounds that you cannot purchase from most chemical suppliers.
With all orders, you can order the exact amount of chemical that you need - with no minimum order - in a cost and time effective manner that other suppliers cannot match.
Benzene Physical Properties & Structure
Benzene is probably the most well known compound in organic chemistry, for the hexagonal shaped C6H6 aromatic ring that is the backbone of most organic molecules in existence. Although it has limited use as a molecule in itself, it is used widely as a precursor for many well known chemical compounds in existence today.
Benzene Derivatives
The number of benzene derivatives is incredibly large, varying from simple to complex derivatives that can combine with a wide range of atoms.
Benzene can form simple halogen-containing compounds, with just one halogen displacing a hydrogen such chlorobenzene and iodobenzene, but can form complex derivatives with hydrocarbons, oxygen and nitrogen-containing compounds for example.
The Reactivity of Benzene
The main reason why benzene is so reactive and can form a wide range of complex compounds is due to the unsaturation of the planar ring. This unsaturation is the reason why benzene can undergo substitution reactions, but unlike alkanes, will not undergo addition or reduction reactions.
The substitution reactions that benzene undergoes are electrophilic substitutions, which mean that nitration, halogenation, acylation and alkylation are all possible, under the right conditions.
Applications of Benzenes & Benzene Derivatives
The global market for benzene and its derivatives is estimated to be around $35 billion, making it one of the top 20 chemicals in terms of volume. The industrial chemical is widely used across many industries due to its versatility and reactivity to make complex compounds.
Benzene is widely used in industry to produce chemicals and materials, which include plastic and other synthetic materials, paints and coatings, fuels and transport.
Related Article(s)
Iodobenzene: Synthesis, reactions, environmental exposure, safety and applicationsJun 25, 2023
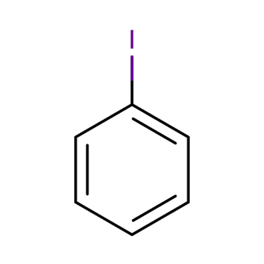
Iodobenzene is an organoiodine molecule that has one of its benzene rings switched out for an iodine atom. Iodobenzene has an empirical formula of C6H5I and a molecular weight of 204.01g/mol. In organic chemistry, it is utilised as an important intermediate in the synthesis process.
Methoxy benzene: synthesis and applicationsJun 18, 2023
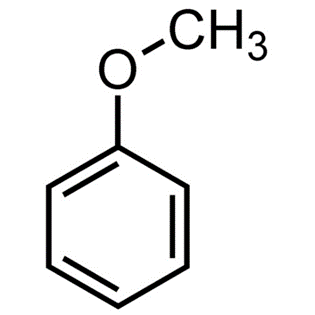
Anisole, commonly known as methoxybenzene, has the molecular formula CH3OC6H5. It does not have an odour, but it has the appearance of anise seed, and some of its derivatives are utilised in fragrances, both natural and artificial. As a scented liquid used in perfume, flavourings, insecticides, and solvents.
Benzene Compounds: Chemical structure and derived compounds Aug 7, 2022
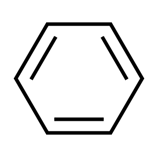
The most fundamental organic chemical is benzene, which belongs to the group of aromatic hydrocarbons. In crude oil, you may find naturally occurring benzene, as well as many other fundamental petrochemicals.
Benzoic Acid: Properties, structure, synthesis, applications and safety hazardJul 14, 2022
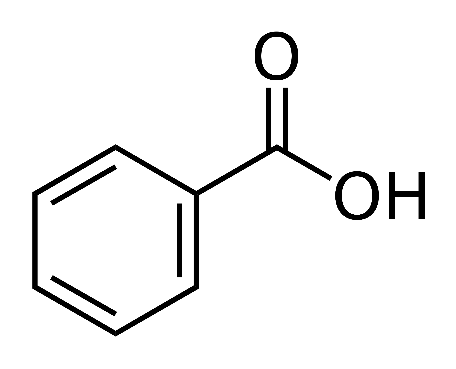
Benzoic acid, an organic molecule with the formula C6H5COOH has its molecular structure composed of carboxyl group that is linked to a benzene ring. This compound is a crystalline, colourless solid at ambient temperature.
Nitrobenzoic acid: Common isomers, application and synthesisJun 30, 2022
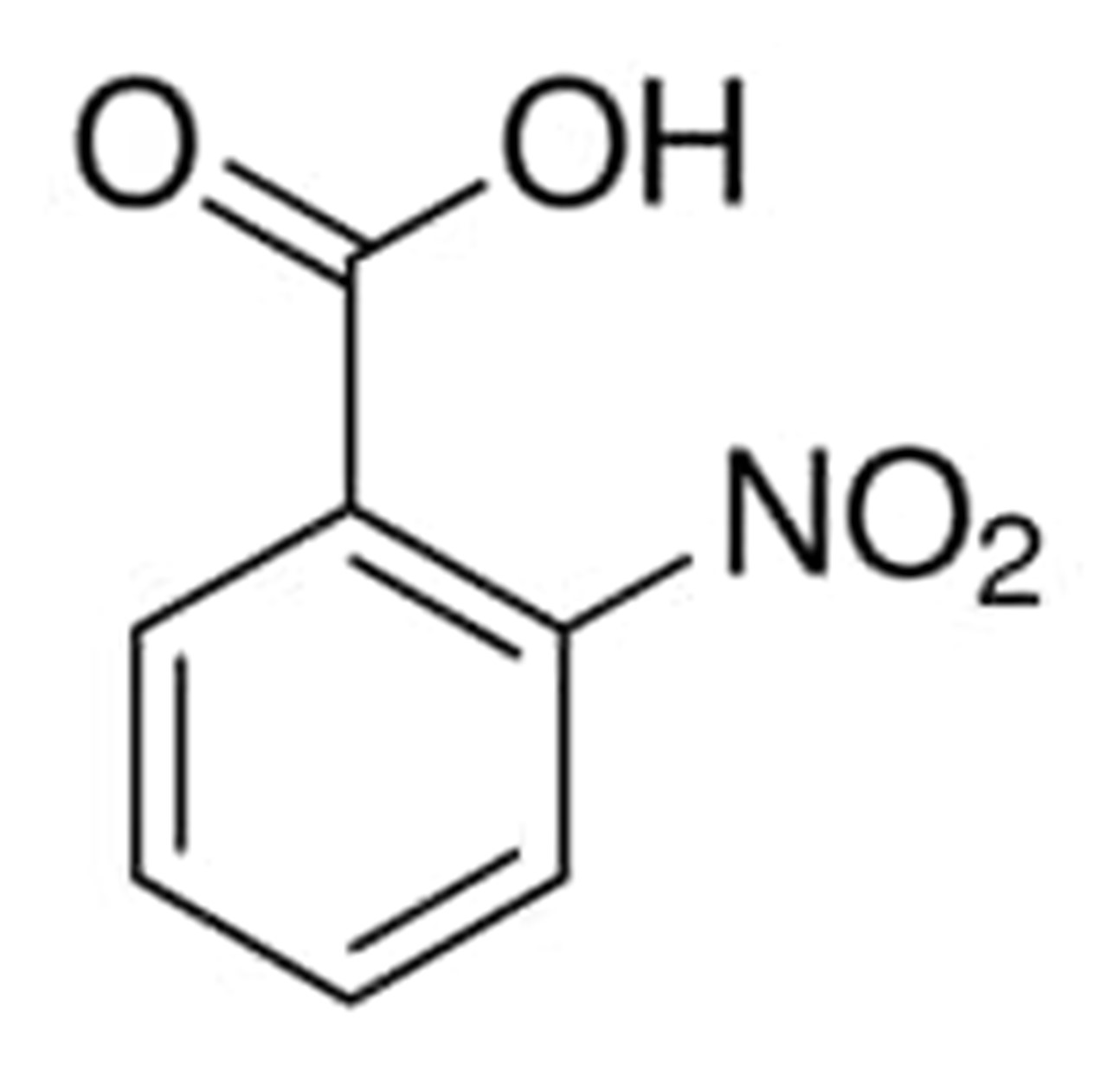
Nitrobenzoic acid is ten times more acidic than the benzoic acid from which they originated. The production of nitrobenzoic acid results from the oxidation of styrene in boiling nitric acid. Nitrobenzoates are the salts and esters of nitrobenzoic acids.