3-chloropicolinic acid (57266-69-0): Synthesis, Applications, Side effects and Storage
Picolinic acid has the formula C5H4N when written down in chemical form (CO2H). It is a pyridine derivative that has had the carboxylic acid (COOH) group replaced. This particular isomer of nicotinic acid, as well as nicotinic acid itself, has the carboxyl side chain located in the third and fourth positions, respectively. It is a white solid that may dissolve in water. It has been used as a substrate in the Mitsunobu reaction as well as the Hammick reaction, both of which belong to the field of synthetic organic chemistry.
3-chloropicolinic acid, a crystallised solid that is off-white in colour and has a melting temperature of around 131 degrees Celsius and a boiling point of 297.6 degrees Celsius, may be beneficial to insecticides, mites, waste fish, and germs. It's possible that the chemical may be used to manipulate the growth of plants as well acting as a strong herbicide.
3-chloropicolinic acid, is also known as 3-chloropyridine-2-carboxylic acid, which is the longer form of its IUPAC nomenclature. It is referred with other names such as 3-chloropyridine derivative carboxylic acid, 3-chloro-2-pyridinecarboxylic acid and 3-Chloro-2-Picolinic acid.
It has the molecular formula of C6H4CINO2 and its molar mass is determined to be 261.0166 g/mol.
According to the Chemical Abstracts Service, the registration number for this substance is 57266-69-0 in the CAS registry.
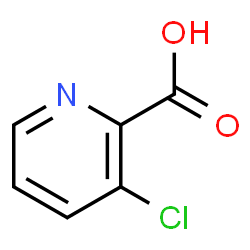
The chemical structure of 3-chloropicolinic acid is as follows:
Synthesis of 3-chloropicolinic acid
The 3-chloropicolinic acid is produced when 3-chloro-2-dichloromethyl pyridine is hydrolyzed by acid. The 3-chloro-2-(trichloromethyl) pyridine may have sulfuric, nitric, or phosphoric acid added to it, and then the mixture can be heated for between half an hour and two hours at temperatures ranging from 20 degrees Celsius to 140 degrees Celsius.
Even though the aforementioned method achieves high yields of the intended output, the completed product's usefulness is restricted because of the high production costs of the intermediate beginning components. This is the case even if the methodology is successful in reducing waste. In order to develop novel procedures, researchers are always looking for raw materials that are both more readily available and more affordably priced.
The following procedures need to be carried out in order to successfully produce 3-chloropicolinic acid:
- The reaction picolinic acid with two to three moles of an alkaline reagent is 3, 5-dichloro-4-hydrazinopicolinic acid. This reaction takes place when picolinic acid is heated.
- The reaction mixture is made acidic using a mineral acid, and the needed amount of 3-dichloropicolinic acid is extracted.
The following are some steps that are performed at various points along this method:
- The 3, 5-dichloro-4-hydrazinopicolinic acid is heated to a temperature of 60° to 150° C or the reflux temperature for approximately half an hour to approximately three hours during the process of carrying out the present invention. During this time, the reaction with an excess of a base is accompanied by agitation.
- At this point, the base may either be hydrazine or an alkali metal hydroxide present in the form of an aqueous solution. A solution of a lower alkanol in water or an aqueous solution of a lower alkanol are also acceptable alternatives. Food may include methanol, ethanol, propanol, isopropanol, and butanol, which are all examples of lower alkanols. Butanol is also possible. Examples of alkali metal hydroxides include sodium hydroxide, potassium hydroxide, cesium hydroxide, lithium hydroxide, and rubidium hydroxide.
- Through the process of crystallisation, it is feasible to retrieve the base salt of the 3-dichloropicolinic acid product that was produced in the prior step. In the great majority of instances, it is better to recover the acid form of the substance. When this occurs, the reaction mixture is brought to a cooler temperature and subjected to the standard acid hydrolysis conditions.
- In order to guarantee that the material is completely converted, acidification is often performed to a pH of roughly 1 to 2 or below. In order to separate the final product, techniques for separating solids and liquids, such as filtering and other more conventional procedures, may be used. In the event that more purification of the acid product is necessary, additional purification may be achieved by washing it in combinations of water, methanol, ethanol, benzene, and hexane.
Applications of 3-chloropicolinic acid
- In rangelands, pastures, and small grain crops, the use of the systemic herbicide 3-chloropicolinic acid is common practice for the management of deep-rooted herbaceous weeds as well as woody plants.
- It is used on pasture and rangeland the majority of the time, with the forestry industry following in a close second.
- The use of products containing 3-chloropicolinic acid in private houses is not permitted under any circumstances.
- Because of the possibility that they may cause damage to plants that are not the intended target, herbicides that include 3-chloropicolinic acid have been classified as Restricted Use pesticides. These products may only be applied by applicators who have been trained and certified.
- When utilizing 3-chloropicolinic acid, which has a variety of regulations about how it may be administered, it is important to observe the 30-day preharvest time for forage and fodder as well as the 7-day pregrazing interval.
- The IOE bans contaminating water that is designed to be used for irrigation or residential use, applying to snow or frozen ground, and applying 2 in close proximity to precious trees if root damage cannot be accepted.
- The use of 3-chloropicolinic acid is subject to certain limitations, such as preventing animals from grazing or feeding on treated areas for a period of two weeks after treatment and preventing the harvesting of hay from grain fields that have been treated. In addition to this, it is not allowed to be used in any kind of irrigation system.
Side Effects of 3-chloropicolinic acid
Because it has acute oral toxicity, taking it by mouth might put your health in jeopardy. Inflammation of the skin is a possibility. It causes the eyes to become so inflamed that they hurt.
It's probable that it'll make your lungs feel uncomfortable. A single dosage may be toxic to specific organs; irritation of the respiratory tract may also occur.
Storage and Sensitivity
3-chloropicolinic acid must be stored at an ambient temperature.