Alcohols: Classification, sources, commercially significant types and uses
Alcohols are chemical substances that include an aliphatic carbon atom with a hydroxyl (OH) functional group attached. Because all alcohols share OH, we usually write alcohols as the general formula ROH, where R is an alkyl group. Alcohol is commonly found in nature. The alcohol in alcoholic beverages is known as ethyl alcohol (ethanol) and it is a member of a big family of chemical compounds known as alcohols. This family also includes common compounds such as cholesterol and sugar. The alcohol homologous series starts with methanol (CH3OH) and progresses to ethanol (CH3CH2OH).
Alcohols are chemically represented as follows:
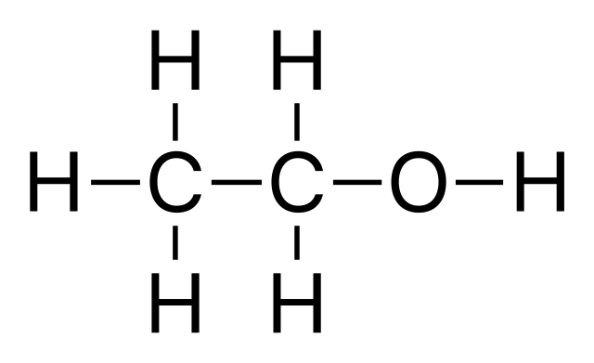
Alcohols with low molecular weights dissolve easily in water, but alcohols with larger molecular weights are less soluble and have higher vapor pressures, boiling temperatures, viscosities and densities,. Most alcohols are transparent liquids at normal temperature. Alcohols with pleasant fruity fragrances, such as isopropyl alcohol, ethyl alcohol and methyl alcohol, are all liquid at ambient temperature. Higher alcohols, especially those with 4-10 carbon atoms, have a thicker viscosity and a fruitier aroma. At room temperature, many alcohols with more than 12 carbon atoms, particularly certain highly branched alcohols, are solids.
Classification of Alcohols
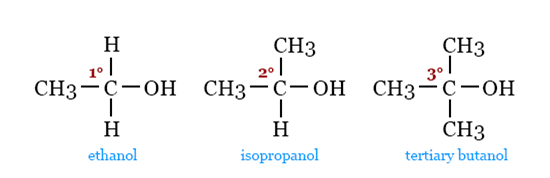
We look at which carbon of the alkyl group is connected to the hydroxyl group to distinguish between primary, secondary, and tertiary alcohols.
If the OH group on carbon is only bonded to one other carbon atom, it is primary (1°) alcohol. The general formula is RCH2OH.
Two more carbon atoms hold the OH group on carbon in a secondary (2°) alcohol. R2CHOH is the chemical formula.
Tertiary (3°) alcohols are those that have three covalent bonds between the OH group and carbon. Its general formula is R3COH.
Sources of Alcohols
Steam distillation is frequently used to extract alcohols with more complicated molecular structures from plant volatile oils. Volatile oils are extracted by boiling plant materials in water and then collecting the condensed vapor containing the oils. Natural alcohols include cholesterol, which is found in most animal tissues (and in huge quantities in egg yolks), and retinol (vitamin A alcohol), which is generated from fish liver oils.
Commercially Significant Alcohols
Methanol
Previously, methanol was produced by heating wood chips without the addition of oxygen (methyl alcohol). The breakdown of part of the wood's carbohydrates into methanol leads in the condensation of methanol vapor. Following this process, methanol was dubbed "wood alcohol." At high temperatures and pressures, commercial methanol is created by the catalytic reaction of carbon monoxide (CO) and hydrogen gas (H2).
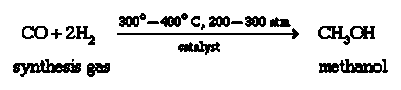
Ethanol
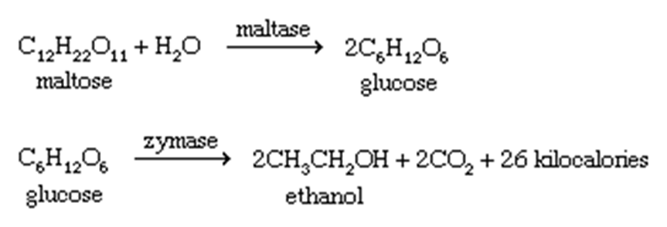
People have been fermenting fruit juices to produce ethanol since prehistoric times (ethyl alcohol). If maintained in an airtight container, wine prepared from fermented juice may be securely stored over the winter. Fermentation converts sugars and starches from various sources into simpler molecules. Ethanol, often known as grain alcohol, is frequently produced by distilling cereal grains such as wheat, corn, rye, and barley. After cooking the grain in water to form mash, malt is added and the combination is allowed to ferment to make wort. Malt contains diastase, an enzyme required for the conversion of grain starches into maltose, the sugar utilized in brewing. During the incubation phase, brewer's yeast is added to the wort; this yeast generates the enzymes maltase and zymase, which break down the glucose and maltose in the wort and convert the glucose to ethanol. The yeast cells obtain energy by converting glucose, a sugar composed of six carbon atoms, to carbon dioxide.
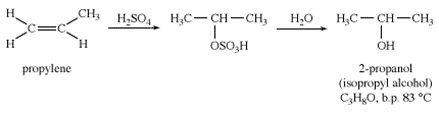
Isopropyl Alcohol
Propylene hydration results in isopropyl alcohol, whereas indirect hydration results in 2-propanol (CH2CHCH3). Isopropyl alcohol is a flexible solvent and antibacterial that may be used in the workplace and on the skin. Isopropyl alcohol is more toxic than ethanol, but it also has a gentler drying effect on the skin and is not subject to the same regulatory scrutiny and taxes in the United States.
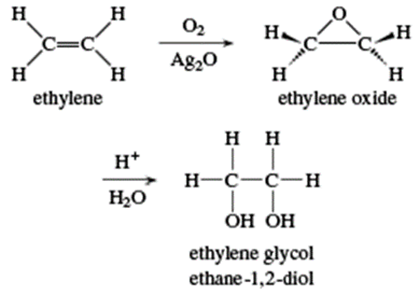
Ethylene Glycol
Ethane-1,2-diol is the technical name for ethylene glycol, which literally translates to "the glycol created from ethylene." Ethane glycol is a component of automotive antifreeze, hydraulic fluid, printing ink, and paint solvent. It is also an important component in the synthesis of polyesters, explosives, alkyd resins, and synthetic waxes.
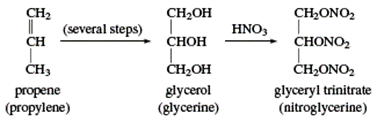
Glycerol
Glycerol (glycerine) is a sweet and syrupy alcohol with three hydroxyl groups. In the chemical world, it is known as propane-1,2,3-triol. In the beginning, saponification (hydrolysis in base) of fats produced glycerol as a byproduct. Each tonne of soap produces around 25 kg (60 lb) of glycerol. Molasses and sugar fermentation produce the same chemical. During World War II, large volumes of glycerol were necessary for the manufacturing of glyceryl trinitrate (nitroglycerin), hence propylene (CH2=CHCH3) was utilised to synthesize glycerol.
Uses
It's no secret that alcohols are common in the realm of organic compounds. Because of their many uses, they are among the most commonly synthesized organic molecules. Among the many alcohols, ethanol and methanol are perhaps the most frequent. Ethanol is found in many ordinary home products, fuels, and medicinal supplies to mention a few of its numerous applications. It's also what gives alcoholic beverages their particular flavour. Aside from being used as an anaesthetic, ethanol is also an essential component in the manufacturing of ether. Methanol may be used as a solvent, a raw ingredient in the manufacturing of formaldehyde and special resins, a component in specialty fuels and antifreeze, and a metal cleanser.