Amines: Synthesis, classification, biochemical significance, applications and hazards
Amines are the compounds that have nitrogen atoms with a lone pair. Alkylamines and arylamines get their names from the fact that they are made by replacing one or more hydrogen atoms in ammonia (NH3) with an alkyl or aryl group.
The smells of the lower-molecular-weight amines range from fishy to rotten. They are either gaseous or vaporized respectively when they are at room temperature or when they are heated quickly. The densities of aliphatic amines, which range from 0.63 to 0.74 g/cm3, are lower than that of water, but the densities of aromatic amines, like aniline (1.02), are often much higher. As they get bigger, they become less volatile, and their smell fades until it can't be detected. However, some diamines have unpleasant smells.
Alkaloids in plants, catecholamine neurotransmitters (like dopamine, epinephrine, and norepinephrine), and the chemical mediator histamine, which is found in the tissues of most animals, are all types of amines. Aniline, ethanolamines, and other amines are important industrial goods that are used to make, among other things, rubber, dyes, medicines, synthetic resins, and fibers.
Occurrence
Amines are found naturally in things like proteins, vitamins, hormones, etc., and they can also be made in a lab to be used in things like plastics, drugs, and dyes.
Synthesis
Aliphatic amines are made in large quantities by chemical processes. The most common industrial process is to mix alcohols and ammonia at high temperatures, usually with metal or metal oxide catalysts like nickel or copper. So, different kinds of amine mixtures are made, such as primary, secondary, and tertiary. In a catalytic reaction, hydrogen is added to nitriles, RCN, to make amines. These amines are then used to make nylon-6, 6.
Aromatic amines like aniline used to be made from coal tar, but now they are made from benzene, C6H6, or other hydrocarbons. In order to change benzene, the first step is to make nitrobenzene (C6H5NO2) or chlorobenzene (C6H5Cl) (C6H5Cl). When treated with hydrogen over a catalyst or with additional reagents like iron or hydrogen sulphide (Fe and H2S, respectively), the former becomes aniline (Fe and H2S, respectively). Aniline is made by heating chlorobenzene and ammonia together in the presence of copper compounds.
Classification
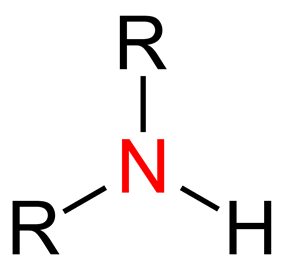
Based on how many hydrogen atoms have been replaced by organic molecules, amines are put into three groups: primary, secondary, and tertiary. The molecular symbols RNH2, R2NH, and R3N are used to describe each of these three types. Amines are basic because they have a nitrogen atom that doesn't make a bond. In chemistry, amines are put into groups based on how many carbon atoms surround the nitrogen atom.
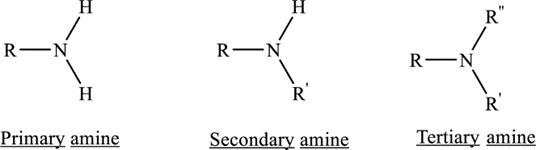
- In primary amines, one of ammonia's three hydrogen atoms is replaced by an alkyl or aromatic group.
- Secondary amines have one hydrogen bonded to the nitrogen and two organic substituents, such as alkyl or aryl.
- In tertiary amines, nitrogen is linked to three organic groups, such as alkyl and aryl.
- Monoamines are made up of a single amino group attached to an aromatic ring by a two-carbon chain. They are found in neurotransmitters, neuromodulators, and hormones. Dopamine, noradrenaline, adrenaline, octopamine, serotonin, and melatonin are all examples of neurotransmitters.
- All four hydrogen atoms of the ammonium ion, NH4+, are swapped out to make quaternary ammonium compounds. An anion must be added (R4N+X) to make the compounds work.
Aliphatic amines don't have any aryl groups attached to them, but aromatic amines do. They can be either aliphatic, where the nitrogen is not part of any rings, or cyclic, where the nitrogen is part of a ring (generally aliphatic).
Biochemical Significance
- Amines are an important part of metabolic and physiological processes in living things.
- Polyamines are important for reproduction, growth, regrowth, and metabolism. They change how permeable and stable cell membranes are and take part in almost every step of the process of making DNA, RNA, and proteins.
- It's safe to say that polyamines are necessary for all living things to work. That's why they're so important, whether you're healthy or sick. When a child is growing and changing quickly, he or she needs more polyamines. Polyamines are important for renewing cells and balancing the effects of hormones and growth factors. People who are otherwise healthy shouldn't have too much of them in their bodies.
- Polyamines are essential for repairing damage to the gut caused by harmful substances in food or bacteria and for keeping the high metabolic activity that is needed for the gut to work normally.
Uses
In many ways, amines are important for human beings. Here are some ways that amines can be used:
- Amines are used for a wide range of things, such as cleaning water, making medicines, and researching and developing insecticides and herbicides.
- Amines help make amino acids, which are the most important building blocks of proteins in living things. Amines also play a part in making some types of vitamins.
- Serotonin is an amino acid that is one of the most important neurotransmitters. It does a lot of different things in the brain. It also affects how hungry you feel and is essential for keeping your brain working at its best speed.
- Painkillers like morphine and demerol, which are analgesics, are made from amines.
Hazards
Simple amines with a low molecular weight, like ethylamine, have an LD50 of between 100 and 1000 mg/kg. Some of them are easy to absorb through the skin, which means they can cause a lot of irritation. [5] In the amine family, there are a lot of different compounds, and some of the more complicated ones, like strychnine and heroin, are very bioactive.