Aminopyridine: Common isomorphs, synthesis, applications and safety
The name "aminopyridine" refers to the amine compound that is derived from pyridine. It has the chemical formula C5H6N2 and its physical appearance is a white solid with a molecular mass of 94.1146 g/mol. It does not dissolve in organic solvents that are polar. It melts at 311 and 316 F0 (428 and 431 degrees Kelvin), and it boils at 273 degrees Celsius (523 degrees Fahrenheit; 546 degrees Kelvin).
Most common isomeric forms of aminopyridine listed below:
- 2-Aminopyridine
- 3-Aminopyridine
- 4-Aminopyridine (4-AP), which is often referred to as fampridine or dalfampridine,
These isomeric forms can be discussed as following:
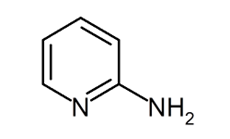
2-Aminopyridine
The chemical formula for 2-aminopyridine is H2NC5H4N. It is one of the three possible isomers of the aminopyridine compound. This odourless solid is used in the production of piroxicam, sulfapyridine, tenoxicam, and tripelennamine, among other drugs. The Chichibabin reaction, in which sodium amide is reacted with pyridine, is the one that results in the synthesis of 2-aminopyridine.
The chemical formula for aminopyridine is presented below in the following diagram:
Uses
2-Aminopyridine is a simple moiety that has a low molecular weight and is fully functionalized that may be used in the synthesis of a number of different biological molecules. A significant number of pharmaceutical companies all over the world are now working on the synthesis of low-molecular-weight compounds with the intention of employing them as pharmacophores against a variety of biological targets. An ideal technique is to use 2-aminopyridine as a locomotive in the manufacture of these compounds and in pulling them towards their respective pharmacological objectives.
Because of the simple nature of this moiety's synthesis, it may be utilized in the manufacturing of individual products while causing the bare minimum quantity of adverse consequences. As a result of the low molecular weight of synthetic compounds, it is also possible to detect metabolites that are responsible for toxicity in drug discovery systems.
Toxicity
The LD50 for acute poisoning is 200 mg/kg (rat, oral).
3-Aminopyridine
The boiling point of 3-aminopyridne is 478 degrees Fahrenheit (248 degrees Celsius), whereas its melting point is 65 degrees Celsius (338 degrees Kelvin). This chemical may be dissolved in a variety of solvents, including water, alcohol, and benzene.
Synthesis
In order to make 3-aminopyridine, nicotinamide is heated in the presence of sodium hypobromite, which is formed locally by the reaction of sodium hydroxide and bromine at a temperature of 70 degrees Celsius.
Uses
This chemical has the potential to be used in the manufacture of the organic ligand 3-pyridylnicotinamide. In addition to that, it is a necessary component in the process of synthesis Troxipide.
4-Aminopyridine
The structure of 4-aminopyridine, commonly known as fampridine or dalfampridine, may be written as C5H4N–NH2. It is one of the three isomeric forms of aminopyridine. The investigation of different kinds of potassium channels involves use of it. It has also been used as a drug to treat some of the symptoms of multiple sclerosis, and it is suggested for people who have multiple sclerosis to boost their capacity to walk so that they may better manage their condition. Clinical tests of the phase III variety started in 2008, and the FDA gave its blessing to use of the chemical in January of that same year.
Synthesis
The amine of phenyl-4-amide is converted into 4-phenylethane by the Hofmann rearrangement, which involves the usage of salt. We can create nitrile and then piperdine-amine by beginning with 4-phenylethane as the starting ingredient for the reaction.
Uses
4-AP is an effective medication for use in physiology and biophysics research pertaining to the investigation of potassium conductance. It is possible to selectively block voltage-activated K+ channels that are members of the Kv1 (Shaker, KCNA) family. On the other hand, it has been proven that 4-AP can increase the activity of voltage-gated Ca2+ channels, and this action is independent of the effects that 4-AP has on voltage-activated K+ channels.
Acts as convalescent
In animal models of epilepsy, the potent convulsant 4-aminopyridine is employed as a test substance for testing the anticonvulsant medications.
Acts as pesticide
Under the trade name Avitrol, 4-aminopyridine is also made available as a bird control bait containing either 0.5 percent or 1 percent of the active ingredient. It is possible that it might induce convulsions or even death, depending on the dose. The correct dosage is supposed to cause convulsions similar to those experienced by people who are epileptic, which results in distress calls from the poisoned birds and their flocks fleeing at once; however, if a sub-lethal dose was used, the birds will recover within four or more hours, and it will not have any long-term impact on their health.
Due to the fact that just a tiny percentage of birds are being killed by the poison, the majority of the flock will be driven away, resulting in a lower overall population. The majority of people will pass away within an hour after consuming a lethal dose. The Humane Society of the United States has taken a stance against the usage of 4-aminopyridine for the purpose of controlling birds.
Medicinal use
Therapeutic use of fampridine have been demonstrated in patients suffering from Lambert-Eaton myasthenic syndrome and multiple sclerosis. It does this by blocking voltage-gated potassium channels, which in turn lengthens action potentials, which in turn leads to an increase in neurotransmitter release at the neuromuscular junction.
The utilization of the medicine has shown in both tissue and animal investigations that it is capable of reversing the harmful effects. In humans, an excess of calcium entry blockers can cause the cytosolic calcium concentration to rise when 4-aminopyridine is present since this compound has the potential to do so.