Applications of Bromohexanoic acid
Bromohexanoic acid in the synthesis of alkyl ketones
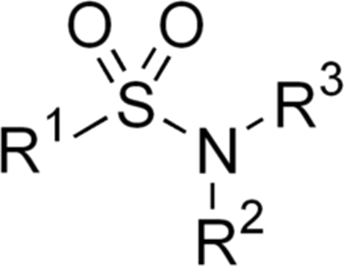
Tosyl isocyanate and alkyl iodides and strontium metal were utilized to convert 6-bromohexanoic acid into N-acyl sulfonamides, which were then employed in the synthesis of alkyl ketones.
Production of 2-lithio-1, 3-dithian
Additionally, it was utilized in the production of 2-lithio-1, 3-dithian, diaryl thioethers, and anionic mitomycin C-dextran conjugate (MMC-D).
Pigment aggregation inhibited by bromohexanoic acid
Synergists PY74ACA-1 and PY74ACA-2 were developed for use in dispersing pigment in water-based ink. As a result of their interaction with pigments and dispersants, synergists boost the degree to which pigment particles are dispersed and stabilize them in this dispersed form. Pigment-74 now has a hydrophilic functional group composed of 6-bromohexanoic acid. Neither water nor organic solvents like DMSO, DMF, or methanol were able to dissolve the original pigments. It was discovered that the solubility of two synergists is more than that of pigment; in particular, PY74ACA-2 is more soluble than PY74ACA-1. Based on these findings, it appears that 6-bromohexanoic acid effectively avoids pigment aggregation and, by increasing polarity, boosts solubility in water. The addition of two synergists to pigment inks should enhance the inks' dispersion qualities and storage stability.
Synthesis of N-Acyl sulfonamides
N-Acyl sulfonamides, diarylthioethers, and 2-lithio-1,3-dithian are all compounds that may be synthesized from 6-bromohexanoic acid. It has a crucial role as an intermediate in organic synthesis.
Bromohexanoic acid as immune-modulator: A study
Primary biliary cirrhosis is characterized by autoantibodies directed against lipoylated enzymes like the E2 component of the pyruvate dehydrogenase complex (PDC-E2), with lipoic acid itself constituting a component of the dominant auto-epitopes. Immune detection of neo-antigens produced by xenobiotic replacement of normal proteins is a potential mechanism for the onset of tolerance breakdown in this illness. Crucially, the xenobiotic 6-bromohexanoic acid (6BH), a lipoic acid analogue, can be used for sensitization with proteins to elicit an immune response that cross-reacts with PDC-E2. Lipoate activating enzyme and lipoyl-AMP (GMP):N-lysine lipoyl transferase are recombinant lipoylation enzymes that have the ability to aberrantly integrate xenobiotic into PDC-E2. These enzymes were shown to be capable of incorporating xenobiotic 6BH and lipoic acid equivalents including octanoic and hexanoic acids into PDC-E2. Activation by adenosine triphosphate (ATP) or guanosine triphosphate (GTP) had varying effects on the efficiency of incorporation of these analogues, with ATP favoring incorporation of hexanoic acid and 6BH and GTP preferring replacement by octanoic acid. Importantly, competition tests revealed that the ratio of ATP to GTP may control the relative incorporation of 6BH and lipoic acid, with 6BH-substituted PDC-E2 synthesis predominating in an ATP-rich environment. Researchers have demonstrated, using a well-defined system in vitro, that an important xenobiotic may be incorporated into PDC by the exogenous lipoylation system in place of lipoic acid; the relative quantities of lipoic acid and xenobiotic incorporation may be regulated by the balance between ATP and GTP. Taken together, findings point to a specific process by which an auto-immunogenic neo-antigen relevant to the pathophysiology of primary biliary cirrhosis is generated.
Bromohexanoic acid role in enzymatic breakdown
Candida rugosa (CRL) and Pseudomonas sp. (PSL) lipases immobilized on poly(ethylene oxide) (PEO) were investigated for their potential synthetic uses. Microscopy and thermal analysis methods were used to investigate the shape and thermal characteristics of the immobilized lipases on the support. The reaction of 5 mmol lauric acid and 5 mmol n-pentanol was tested to determine the efficacy of these systems. Time, heat, enzyme concentration, and n-hexane, an organic medium, were all tested to see how they affected the reaction. Esterification progressed more rapidly with increasing time, temperature, and concentration. For this process involving a CRL/PEO system, the effect of organic solvents (n-hexane, cyclohexane, n-heptane, and acetone) was also studied. Studies were also conducted on the enzymatic breakdown of (R,S)-2-octanol, (R,S)-2-(4-chlorophenoxy) propionic acid, and (R,S)-2-bromo hexanoic acid. Enantiometric excess of product (eep) >99% (E=180) was achieved in the production of the R-alcohol. Racemic acid resolution yielded enantiomeric excess for the substrate (ees) values of 1-76 percent and enantiomeric excess for the product (eep) values of 6-96 percent (E=1, 1-118).
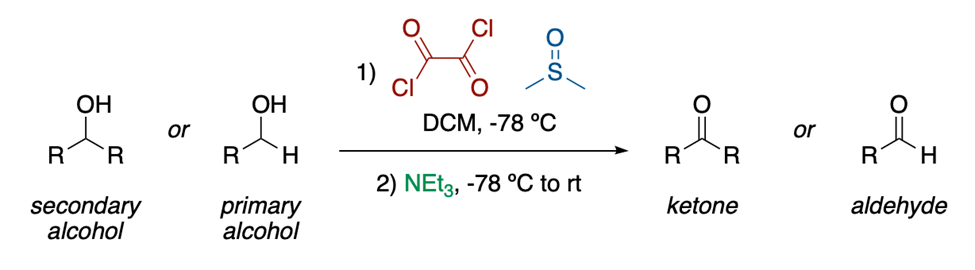
Bromohexanoic Acid in Swern oxidation processes
Accessible in a timely manner Swern oxidation processes with oxalyl chloride smoothly convert primary or secondary alcohols to matching aldehydes or ketones with high yield, and 6-(methylsulfinyl)hexanoic acid is used as a replacement for DMSO in these reactions. The resultant 6-(methylthio) hexanoic acid may be reoxidized to 1 using sodium metaperiodate in a yield of 97%, and it is easily purified through water extraction or filtering through silica gel. The intermediates of this Swern process are studied by 13C NMR spectrometry at very low temperatures (60 °C). The results show that any unoxidized alcohol remains after Pummerer elimination of the alkoxysulfonium intermediate and may be reduced by prolonged exposure to triethylamine at 40 °C. A polymer-bound reagent 12 is obtained by reacting the potassium salt of 1 with cross-linked chloromethyl polystyrene. This reagent oxidises borneol to camphor in a quantitative fashion when applied in a twofold excess.
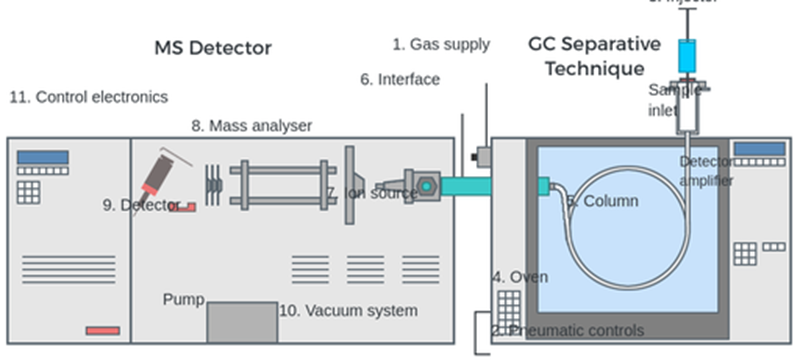
Bromohexanoic acid as an internal standard in (GC-MS)
After purification on an anion-exchange cartridge, t,t-MA was derivatized with BF3-methanol to the dimethyl ester and evaluated by gas chromatography-mass spectrometry (GC-MS) using 2-bromohexanoic acid as an internal standard. This approach was shown to be sensitive and specific. The recovery rate varied between 93.3% and 106.3%, the limit of detection was 0.01 mg/l, and the coefficient of variation for duplicate analysis in a series of urine samples (n = 50) was 2.6%. The analysis has a 7.4% daily accuracy and a 14.6% daily precision between days. The procedure was used to quantify t,t-MA in the urine of both smokers and nonsmokers. Ten smokers had a mean t,t-MA concentration of 0.09 0.04 mg/g creatinine, which was statistically (p = 0.012) greater than the t,t-MA concentration in the urine of ten nonsmokers, which was 0.05 0.02 mg/g creatinine. No interference between t,t-MA and other urinary chemicals was identified, in contrast to results obtained with the routinely used high-performance liquid chromatography ultraviolet detection (HPLC-UV) techniques. The biomonitoring of low-level benzene exposure in the environment is facilitated by this GC-MS technique since it is both selective and sensitive.