Applications of Chlorocinnamic Acid
The plant hormone 4-Chlorocinnamic acid (C442030) is derived from the cinnamic acid (C4420). Potential antibacterial, antioxidant, anti-inflammatory, and antitumor action of 4-chlorocinnamic acid has been reported.
Chlorocinnamic acid showing antifungal activity: A study
Resistance to antimicrobial treatments in fungal and bacterial infections is now recognized as a serious threat to public health. Cinnamic acid esters offer a wide range of pharmacological effects, including antibacterial ones. Fischer esterification, alkyl or aryl halide esterification, and the Mitsunobu and Steglich reactions were utilized to synthesize a series of structurally similar 4-chlorocinnamic acid esters. Esters were tested for their antibacterial effects against several strains of bacteria and yeast, including those of the species Candida albicans, Candida glabrata, Candida krusei, Candida guilliermondii, Pseudomonas aeruginosa, and Staphylococcus aureus. Furthermore, a molecular docking analysis was performed on the chemicals and the enzyme 14-demethylase. From 4-chlorocinnamic acid, researchers were able to generate a total of twelve esters with yields ranging from 26.3% to 97.6%; three of them are reported here for the first time. One study found that even at the highest dose tested, the ester methyl 4-chlorocinnamate showed efficacy against S. aureus. Methoxyethyl 4-chlorocinnamate and perillyl 4-chlorocinnamate were the most effective esters in the antifungal examination (MIC = 0.13 and 0.024 mol/mL, respectively).
Chlorocinnamic Acid as Anti-oxidant
Cinnamic acid is a naturally occurring organic acid with a wide range of biological activity and minimal toxicity. Derivatives of cinnamic acid are of great interest and promise as potential new drug candidates in the ongoing hunt for novel pharmacologically active chemicals. It is widely believed that the antioxidant capabilities of several derivatives of cinnamic acid, particularly those containing the phenolic hydroxyl group, contribute to a variety of health advantages. The antibacterial properties of cinnamic acid are also widely documented. Antibacterial, antiviral, and antifungal activities in cinnamic acid derivatives have been identified for both naturally occurring and synthetically produced forms of the compound.
Cinnamic acid scavenging ROS
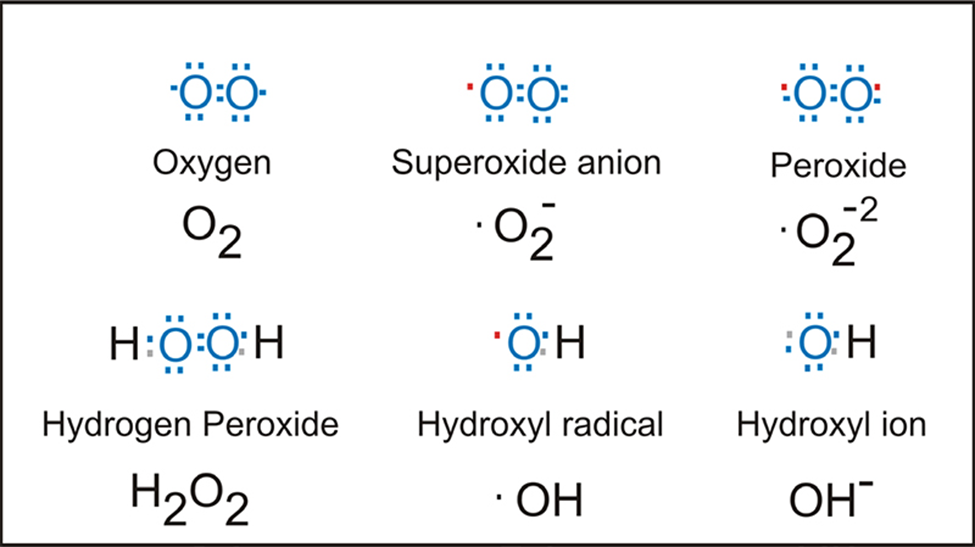
Many laboratories have found the phenolic derivatives of cinnamic acids, including caffeic, ferulic, and sinapic acids, to be promising candidates for use as antioxidant and anti-inflammatory medicines due to their wide range of useful properties. Researchers have also discovered that they are high in antioxidants, have anticancer, cardiovascular, and antibacterial characteristics, and are effective against inflammation, oxidative damage, and oxidative stress. In particular, p-coumaric acid, also known as 4-hydroxy-trans-cinnamic acid, has antioxidant action by directly scavenging reactive oxygen species (ROS) and so reducing LDL oxidation.
P-coumaric acid is said to be a powerful antioxidant and free radical scavenger. Cultured endothelium cells subjected to high glucose and free fatty acid, keratinocytes exposed to UV rays, and lens epithelial cells exposed to hydrogen peroxide have all showed its antioxidant action. Its antibacterial properties manifest themselves through its ability to intercalate the groove in the genomic DNA of bacteria and its ability to damage the membranes of bacterial cells. The antibacterial and antioxidant effects of p-coumaric acid-containing polymers have been shown to help in the healing of skin wounds.
Role of Cinnamic acid in the treatment of Cancer
Both cinnamic acid and its phenolic counterparts occur in nature. Chemically speaking, the 3-phenyl acrylic acid functionality in cinnamic acids provides three primary reactive sites: phenyl ring substitution,-unsaturation addition, and carboxylic acid functionality reactions. These chemical properties have attracted a lot of attention in medical research, both as historical and modern synthetic anticancer medicines derived from cinnamic acid. While cinnamic acid derivatives have a long history of usage in traditional medicine, its anticancer potential has been overlooked for decades. The first recorded clinical use of cinnamic acid derivatives was in 1905. Over the last two decades, researchers have focused extensively on the anticancer properties of different cinnamoyl derivatives. For the first time, this study brings together the vast body of research on the synthesis and biological assessment of numerous cinnamoyl acids, esters, amides, hydrazides, and related derivatives for use in cancer research.
To back up its possible anticancer benefits, p-coumaric acid has been demonstrated to limit the growth and migration of cancer cells while simultaneously encouraging their death via apoptosis. Animal studies have shown that p-coumaric acid can operate as a chemo preventive agent against colon cancer by decreasing inflammatory responses and increasing antioxidant capacity.
Cinnamic acid having antitumorigenic properties
Cancer is the leading global killer by a wide margin. A wide variety of therapeutic applications, including anticancer agents, have benefited from the abundance of medications that may be derived from natural sources. In recent decades, structural optimization of natural products has led to the development of a number of important medications that were previously unavailable. Since cinnamic acid has been shown to have antiproliferative, antioxidant, antiangiogenic, and antitumorigenic properties, it has attracted a lot of attention. Various pharmacological properties, including immunomodulation, anti-inflammation, anti-cancer, and antioxidant, have been observed in cinnamic acid and its analogues, including caffeic acid, sinapic acid, ferulic acid, and isoferulic acid. Many promising new anticancer chemicals have been derived from them.
P-coumaric acid as anti-melanogenic
P-coumaric acid, also known as 4-hydroxycinnamic acid, is a phytochemical that has been linked to several health advantages. It shares a close chemical structure with L-tyrosine, the substrate of tyrosinase in melanogenesis at the cellular level. It was discovered not too long ago that p-coumaric acid is an extremely effective and specific inhibitor of human tyrosinase. Several experimental and human investigations have shown that it has anti-melanogenic properties. Examining the latest research on the biological actions of p-coumaric acid is relevant because of the need for natural skin lightening agents in the cosmetics industry. Based on in vitro research, it appears that p-coumaric acid can suppress the catalytic activity of tyrosinase, hence lowering melanin production. When L-tyrosine was employed as the substrate, p-coumaric acid reduced tyrosinase catalytic activity more effectively than when L-DOPA was utilized. Since p-coumaric acid and L-tyrosine are so chemically similar, it's possible that they battle for access to the same enzyme's active sites.
It is debatable whether or whether p-coumaric acid reduces cellular tyrosinase expression. P-coumaric acid suppressed -MSH-induced tyrosinase protein expression in some investigations, but in others it had no effect on CREB phosphorylation or tyrosinase expression. Not only does L-tyrosine serve as a substrate for the tyrosinase enzyme, but it also plays a hormone-like stimulatory role in tyrosinase gene production. Tyrosinase gene expression is increased by L-tyrosine because it improves the binding capacity of -MSH receptors. p-coumaric acid and L-tyrosine are structurally similar, so it's tempting to think that they might both inhibit L-ability tyrosine's to attach to the MSH receptors' regulatory site.
Cinnamic acid in cosmetics
It was also discovered that applying a lotion containing p-coumaric acid reduced the redness and subsequent pigmentation of human skin caused by exposure to ultraviolet light. Because a control cream void of p-coumaric acid did not exhibit these effects, it was determined that p-coumaric acid was responsible for them in the p-coumaric acid cream. After receiving a complete UV tan, human skin responded favorably to p-coumaric acid cream, demonstrating a depigmenting effect. In order to prevent UV-induced erythema and keep skin a more natural shade of fair, p-coumaric acid can be applied to the skin either before or after time in the sun.
Cinnamic acids as anti-inflammatory
In adjuvant-induced arthritic rats, p-coumaric acid decreased tumour necrosis factor-alpha (TNF-) and macrophage phagocytic index while raising serum immunoglobulin levels, demonstrating anti-inflammatory actions. In addition, it reduced the toxicity of arsenite and doxorubicin to the heart and the toxicity of alcohol and acetaminophen to the liver.