Applications of Nitrobenzoic Acid
Nitrobenzoic acid promotes Glucose oxidation reaction
To improve the efficiency of enzymatic biofuel cells and the glucose oxidation reaction (GOR), it has been proposed to use a novel anodic catalyst composed of carbon nanotubes, 4-nitrobenzoic acid, chitosan, genipin, and glucose oxidase (GOx) (CNT/4-NBA/[Chit/GOx/GP]) (EBC). In this catalyst, numerous GOx molecules are maximally trapped, their leaching out is controlled, and the GOR is enhanced thanks to the cross-linked structure of chitosan and genipin and the correct distribution of amine groups within chitosan. Also, 4-nitrobenzoic acid is an effective mediator. UV-vis spectroscopy, pH tests, and electrochemical characterizations are used to assess the impact of the cross-linked structure. Characterizations show that the new CNT/4-NBA/[Chit/GOx/GP] catalyst contains a high concentration of GOx (17.8 mg/mL) and generates a high anodic current (331 A/cm2 at 0.3 V vs Ag/AgCl) with a low onset potential (0.05 V vs Ag/AgCl) because its catalytic activity follows the desirable reaction pathway that minimizes creation of a proto When the efficiency of an EBC with this catalyst as the anodic electrode is evaluated, the EBC demonstrates a high open-circuit voltage of 0.54 V and a maximum power density of 38 W/cm2.
Nitrobenzoic acid to measure glutathione reductase essay
The glutathione reductase assay, which utilizes the generation of GSH from an excess of GSSG, is explained, along with the usage of 96-well microliter plates and a programmed microplate reader. The samples are read for absorbance at 415 nm against a reference wavelength of 595 every 30 seconds for three minutes. Increase in absorbance velocity is related to glutathione reductase concentration. A standard curve is used to calculate the level of activity in a sample of unknown size. The assay can quickly and simultaneously test two or more duplicates of several tiny samples.
Nitrobenzoic acid in Measurement of serum cholinesterase
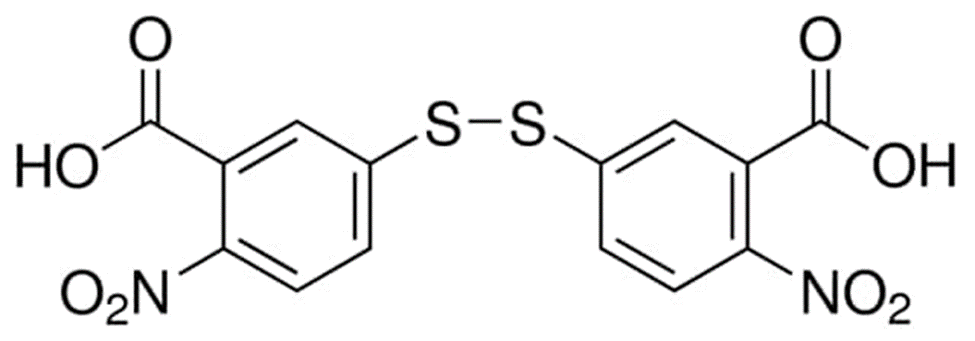
Serum cholinesterase (EC 3.1.1.8, acylcholine acyl-hydrolase) levels have been measured for a variety of purposes, including determining liver function, tracking overexposure to organophosphorus insecticides (which inhibit cholinesterase activity), predicting who will be at risk for experiencing prolonged apnea after taking succinylcholine to relax their muscles, and studying the heritability of cholinesterase variants. Serum cholinesterase may be measured in a number of ways, but the colorimetric approach developed by Garry and Routh that uses the Ellman reaction has lately gained favour due to its ease of use, rapidity, and sensitivity. In order to produce the 5-thio-2-nitrobenzoate ion, cholinesterase first hydrolyzes propionylthiocholine, releasing a free sulfhydryl that interacts with 5,5'-dithiobis-(2-nitrobenzoic acid) (DTNB). DTNB has a maximum absorption at 320 nm, while the yellow nitrobenzoate has an absorption maximum at 410 nm. The conventional process for detecting cholinesterase variations is conducted with and without the inhibitors dibucaine and sodium fluoride.
Mycobacterium showing nitrobenzoic acid susceptibility
P-nitrobenzoic acid sodium salt (m.p. Lowenstein medium) concentrations (0–500 gm./ml). The growth of 29 Mycobacterium TB strains was inhibited by 500 gm/ml, and most strains were killed by 250 gm/ml. These concentrations were effective in suppressing Myco. bovis. Other mycobacteria (1–25 strains) thrived in p-nitrobenzoic acid at all doses, with the exception of 1 photo-chromogen (Bostrum D-35), which was suppressed at 500 ig/ml. For the purpose of determining the species of a mycobacterium, it has been common practice to cultivate the bacteria on media that has been supplemented with inhibitory chemicals. -nitrobenzoic acid (PNB) inhibits the growth of the Mycobacterium tuberculosis complex (MTC), but non-tuberculosis mycobacteria (NTM) are resistant.
Medications for asthma that include nitrobenzoic acid
By releasing the enzyme myeloperoxidase, which catalyzes the creation of the potent oxidant hypochlorous acid (HOCl) from hydrogen peroxide (H2O2) and chloride (Cl), neutrophils engage in bactericidal activity. Higher proteolytic activity in locations of pulmonary inflammation can be caused by HOCl's ability to inactivate 1-antiproteinase (1-AP). Enzyme tests (such as 1-AP and elastase) can be time intensive yet are commonly used in the hunt for potential HOCl scavengers. For this purpose, we designed a procedure wherein HOCl oxidises 5-thio-2-nitrobenzoic acid with relative ease. A compound's ability to scavenge HOCl is determined by how well it prevents this oxidation from occurring. Researchers put a number of well-known HOCl scavengers, including S-methylated glutathione and oxidized lipoate, to the test to demonstrate the methodology. Then, numerous asthma medications were tested and compared. These included terbutaline, isoproterenol, salbutamol, cromoglycate, theophylline, and dexamethasone. Only terbutaline was effective as a HOCl scavenger.
Nitrobenzoic Procaine as anesthetic and skin ointment
A local anesthetic of the ester type is that which has a slow onset and a short duration of action. Infiltration anesthesia, peripheral nerve block, and spinal block are the most common applications for this technique. (Referenced from Martindale's 30th edition of The Extra Pharmacopoeia, page 1016) Oral entry inhibitor studies with procaine have also been performed in individuals with HIV who have previously received therapy. Two common local anesthetics are benzocaine (BC) and procaine (PC) Many pharmaceuticals and medicines rely on them as their active ingredients. Local, infiltration, spinal, and therapeutic blocking are all possible with PC . Generally speaking, BC is most useful when used topically, such as in skin creams, dry powders for skin ulcers, throat lozenges, and teething formulas for small children that are sold without a prescription.
Procaine as local anesthetic
Short-acting ester local anesthetic procaine has been used for spinal anesthesia since the early 20th century, when it superseded cocaine as the medication of choice. Although lidocaine eventually supplanted procaine, the latter's association with transient neurologic symptoms (TNS) has prompted a new look at procaine as a potential fast-acting local anesthetic.
Procaine in anti-fibrillatory and anti-arrhythmic treatment
Direct administration of procaine (a molecule distinct from Procainamide due to the ester structure replacing the amide connection) to the heart raised the threshold of ventricular muscle to electrical stimulation, as shown by Mautz in 1935. This finding was further developed by a large group of researchers, who found that the local anesthetic has cardiac effects similar to quinidine. However, procaine's strong effects upon the central nervous system and its brief duration of action due to fast enzymatic hydrolysis of the substance restrict the medication's therapeutic efficacy as an anti-fibrillatory and anti-arrhythmic treatment. Procainamide was introduced as a cardiac medication after a comprehensive investigation of procaine's congeners and metabolites yielded a molecule with therapeutically effective quinidine-like effects. Multiple methods of administration make the medicine beneficial for treating different types of arrhythmia. Although effective in a variety of contexts, it has a limited window of effectiveness and a high propensity for causing side effects when used repeatedly.