Aromatic Heterocycles: Introduction, classification and applications
Carbon is just one of many different elements that can be found inside the rings of cyclic molecules. A heterocycle is a type of cyclic compound that has more than just carbon in it. Most heterocyclic molecules have carbon and other elements like nitrogen, oxygen, or sulphur. Some heterocyclic compounds are aromatic, so it's important to talk about how the presence of atoms other than carbon changes how aromaticity is judged.
In organic chemistry, compounds that have one or more aromatic rings are called aromatic. "Mono- and polycyclic aromatic hydrocarbons" is one of the many names for them. The main chemical is called benzene. Heteroarenes are similar chemically because the carbon atom in the CH group has been replaced by oxygen, nitrogen, or sulphur. Furan, a heterocyclic compound with a five-membered ring that has one oxygen atom, and pyridine are two non-benzene compounds that have aromatic properties (a heterocyclic compound with a six-membered ring containing one nitrogen atom).
Polycyclic Aromatic Hydrocarbons
Polynuclear aromatic hydrocarbons, which are sometimes called "PAHs," are a type of aromatic hydrocarbon with fused aromatic rings but no substituents or heteroatoms. Naphthalene is an example of a PAH that is easy to understand. Oil, coal, and tar are all natural sources of PAHs, and when they are burned, PAHs are made (whether fossil fuel or biomass). Some molecules in them have been found to cause cancer, genetic changes, and birth defects. This makes them a cause for concern as pollutants. PAHs can be found in both raw and cooked foods, which is important to remember. For example, research has shown that high levels of PAHs can be found in smoked fish and meat that has been cooked at high temperatures, like on a grill or barbecue.
They have been found in comets and meteorites and are thought to be one of the chemicals that set the stage for the first forms of life. Because graphene only has two dimensions, the PAH pattern can be made very big.
Some of the Aromatic Hetrocycles can be discussed in detail as following:
Pyridine
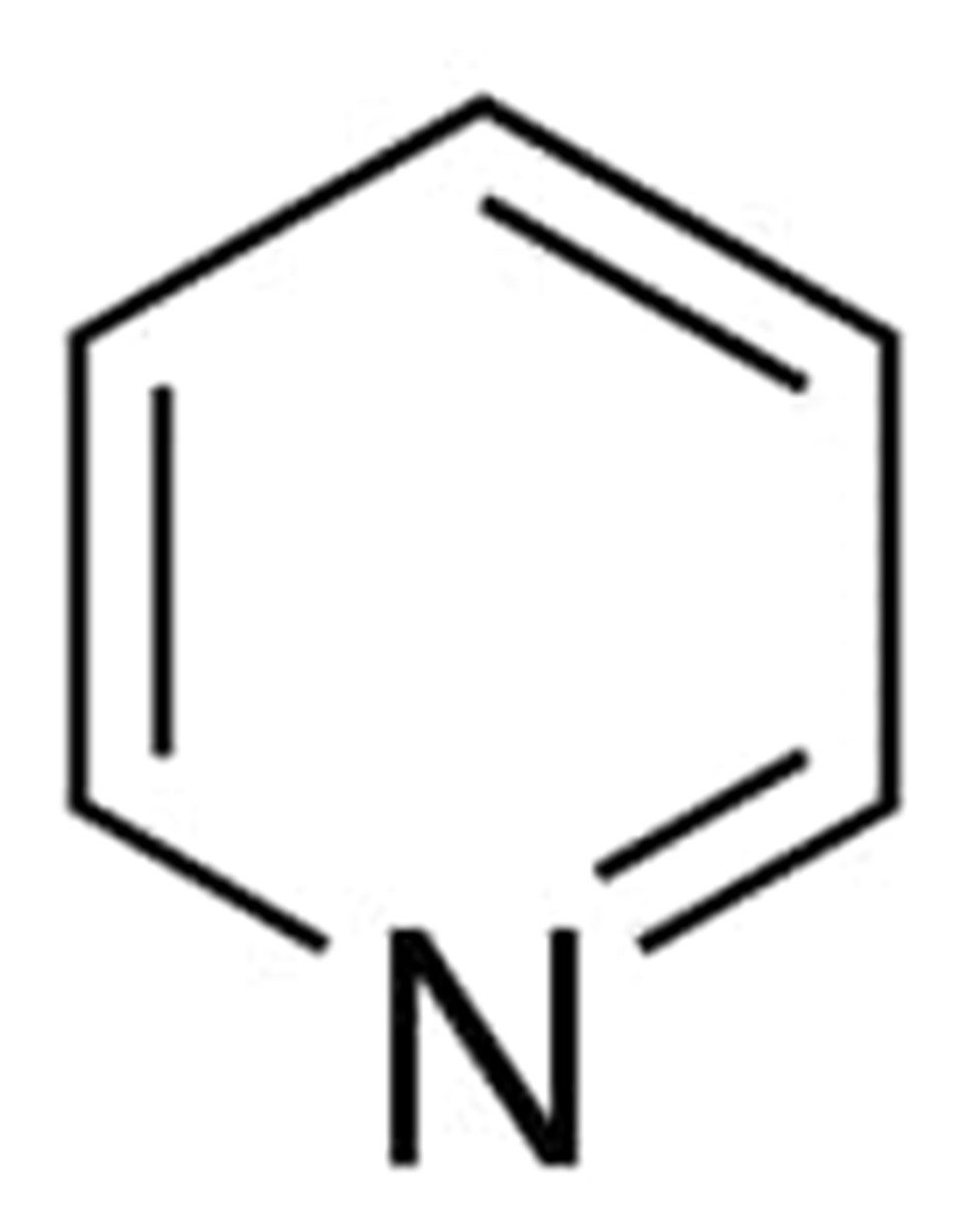
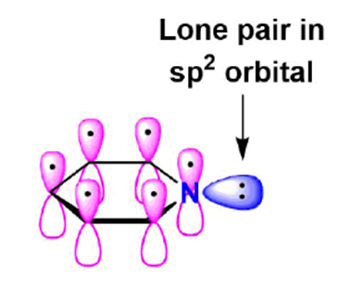
The six carbon atoms in pyridine make it an aromatic heterocycle. In pyridine, the nitrogen atom is sp2-hybridized, which means that two of its sp2 orbitals overlap with those of the carbon atoms next to it, and the third has a lone pair. The single electron in the unhybridized p orbital is one of the six pi electrons that are spread around the ring in a way that is not local.
The lone pair electrons of the nitrogen in pyridine live in a sp2 orbital that shares the plane of the ring but does not touch the p orbitals of the ring. In this case, the orbit is the same as the orientation of the ring. Since lone pair electrons are not part of the aromatic system, their presence has no effect on the number of p electrons.
Instead of being spread out across the ring, the lone pair electrons, which are a color between yellow and red on the electrostatic potential map of pyridine, are attached to the nitrogen.
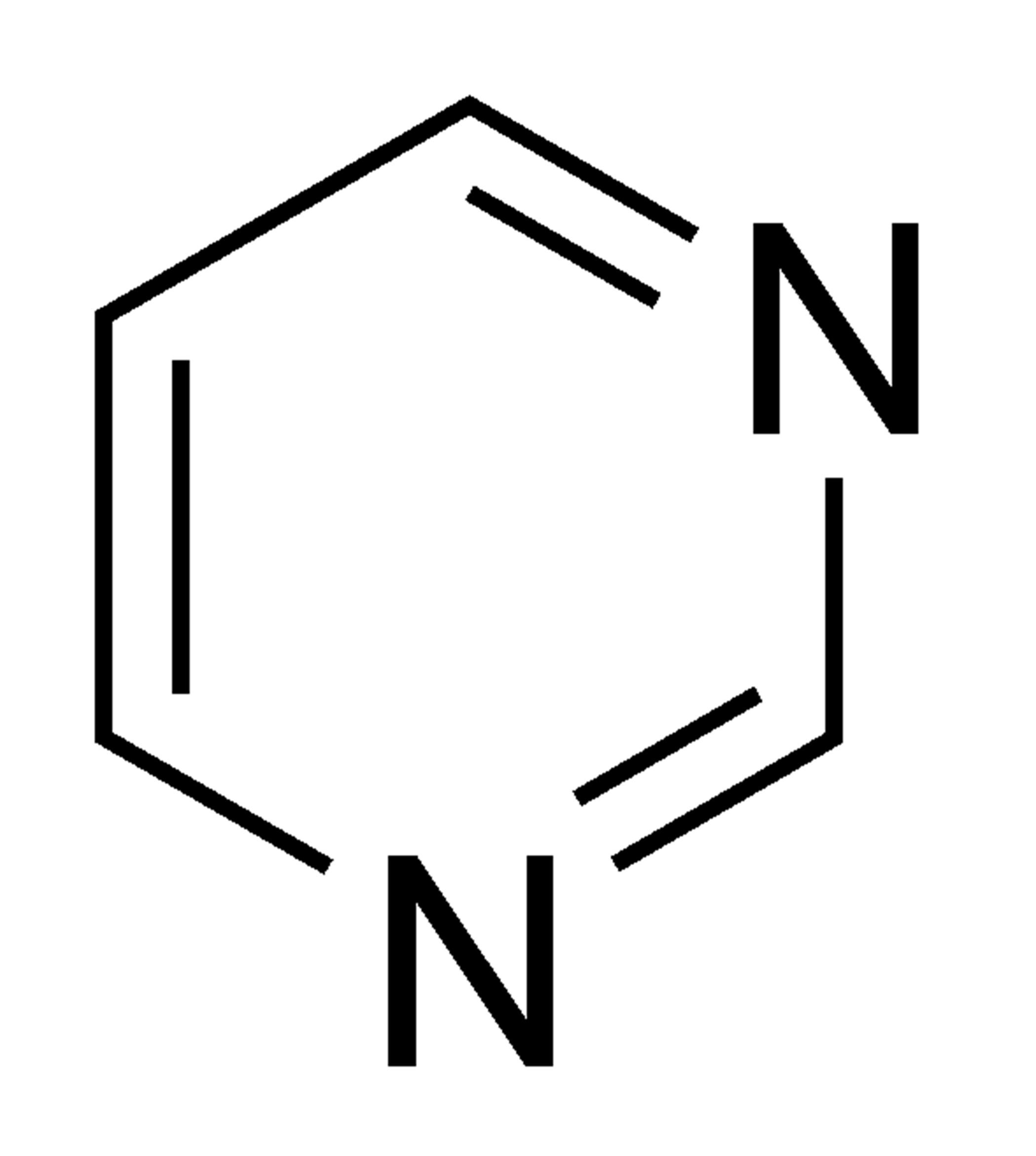
Pyrimidine
The structure of pyrimidine, an aromatic heterocycle, is similar to that of pyridine. Both have six carbon atoms. The sp2 hybridized chemical pyrimidine is made up of four carbon atoms and two nitrogen atoms. Because each atom in pyrimidine has both a p orbital and a pi election, it is possible for it to be both fully conjugated and aromatic. Since both of pyrimidine's nitrogens are filled with lone pairs of electrons in sp2 orbitals, this nitrogen does not take part in the aromatic system. The electrostatic potential map of pyrimidine shows that neither of the sets of lone-pair electrons are spread out over the ring.
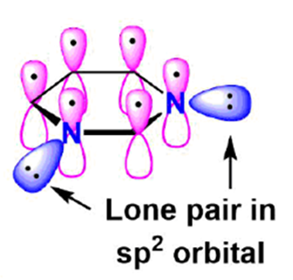
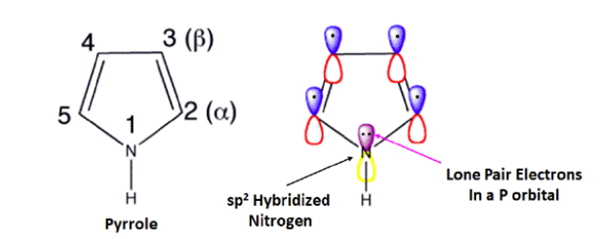
Pyrrole
Five of the six aromaticity-causing pi electrons in pyrrole are in p orbitals, while the other two are in sp orbitals. This makes the heterocyclic ring a true five-membered wonder. Each carbon in pyrrole adds one p orbital and one pi electron. Two of the p electrons in pyrrole come from the nitrogen, which has sp2 hybridised and moved its lone pair electrons into the p orbital. Electrostatic maps of pyrrole show where lone pairs of electrons are in the nitrogen ring. Since the nitrogen lone pair is part of the aromatic sextet, there are fewer free electrons that can connect to a proton. This makes the electrons very stable (and if they do pick up a proton, the molecule loses aromaticity). The pyrrolium ion, which is the conjugate acid of pyrrole, is a strong acid with a pKa of 0.4. This means that pyrrole is a very weak base.
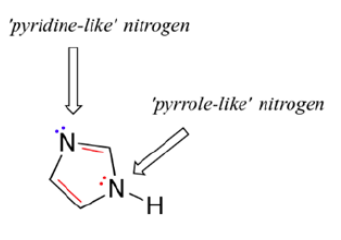
Imidazole
For example, the side chain of histidine has an imidazole ring, which is a well-known example of an aromatic heterocycle found in biomolecules. The two nitrogens in imidazole are very different from each other. Imidazole is aromatic, just like pyrrole, because one of its nitrogen atoms is like pyrrole and gives its lone pair of electrons. The second nitrogen is similar to pyridine because its lone pair electrons are confined in a sp2 hybridized orbital. Because its lone pair electrons can be used to make bonds, imidazole (pKa = 6.95) is more basic than pyrrole (pKa = 0.4).
**Uses **
The usage of heterocyclic compounds is common. Their main uses are in medicine, agriculture, and veterinary medicine. Among other things, they are utilised as disinfectants, developers, antioxidants, corrosion inhibitors, copolymers, and coloring agents.