Aryls: Structure, nomenclature, types and applications
An aryl group is the name given to the functional group that is produced when an aromatic ring complex is reduced to its simplest form by removing one hydrogen atom. The aromatic ring will almost always be constituted by a hydrocarbon in most instances. The names of hydrocarbons are given an additional -yl ending, as in thienyl, phenyl, indolyl and so on. The term "aryl" can be used to refer to any group that falls within the umbrella of the aryl family.
Chemically, an aryl group can be written as following:
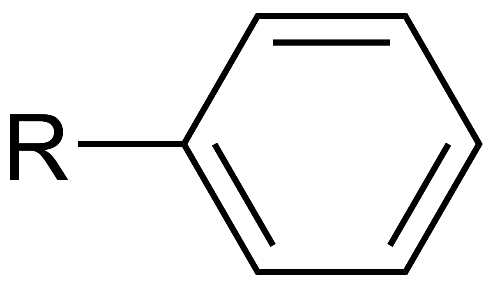
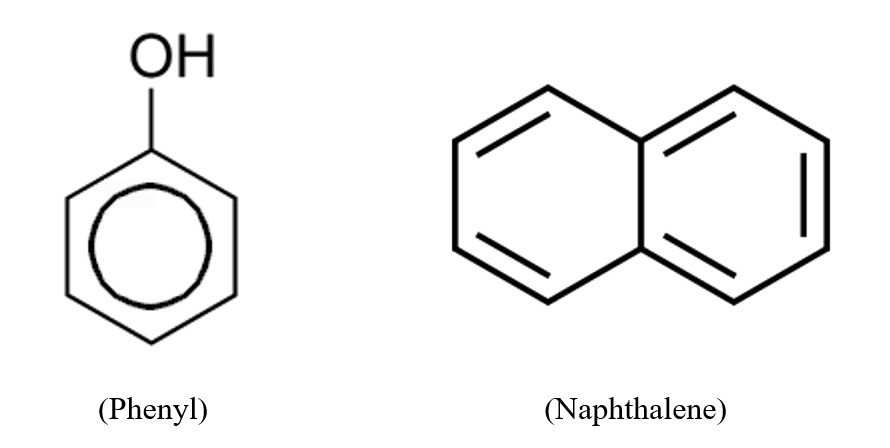
It is usual practice to use the notation "Ar" if there is an aryl present in the structural makeup of a molecule. This is the same as the chemical symbol for argon, but no one is confused by it because the context is organic chemistry, and since argon is a noble gas and hence inert, no one mistakes it for anything else. An aryl group can undergo arylation, which is the addition of a substituent to the group. An illustration of this would be the phenyl (C6H5) functional group, which is an example of an aryl functional group and is formed from benzene. Napththyl is the name given to the aryl group that may be synthesized from naphthalene (C10H7). These two aryl compounds can be chemically written as following:
Nomenclature
The phenyl group, which has the chemical formula C6H5O and is the simplest type of aryl group, is comprised of a benzene ring that has a single hydrogen atom substituted with some other type of substituent. The phenyl group should not be confused with the benzyl group, which is made up of a phenyl group that has been connected to a methyl group and has the formula C6H5CH2.
Phenyl (Hydroxybenzene)
Phenyl groups can be considered to be the parent hydrocarbon, and the suffix "- benzene" can be used to denote compounds whose names contain phenyl groups. One possible meaning of the phrase is that it may also be used to refer to the phenyl group itself as the substituent. When the connected group to the phenyl comprises six carbon atoms or more, this is a frequent method of construction. An example of this would be the combination of a hydroxyl group with a phenyl group. If the phenyl group were to be regarded the parent hydrocarbon, then the appropriate name for this compound would be hydroxybenzene. Another possibility is to consider the hydroxyl group to be the parent group and the phenyl group to be the substituent; this would result in the more common identification of phenol.
Naphthalene
Both the phenyl and the naphthyl groups are considered to be aryl groups. These groups can be produced from either benzene or naphthalene. It is conceivable for there to be substitutions within the aromatic structure of these aryl groups.
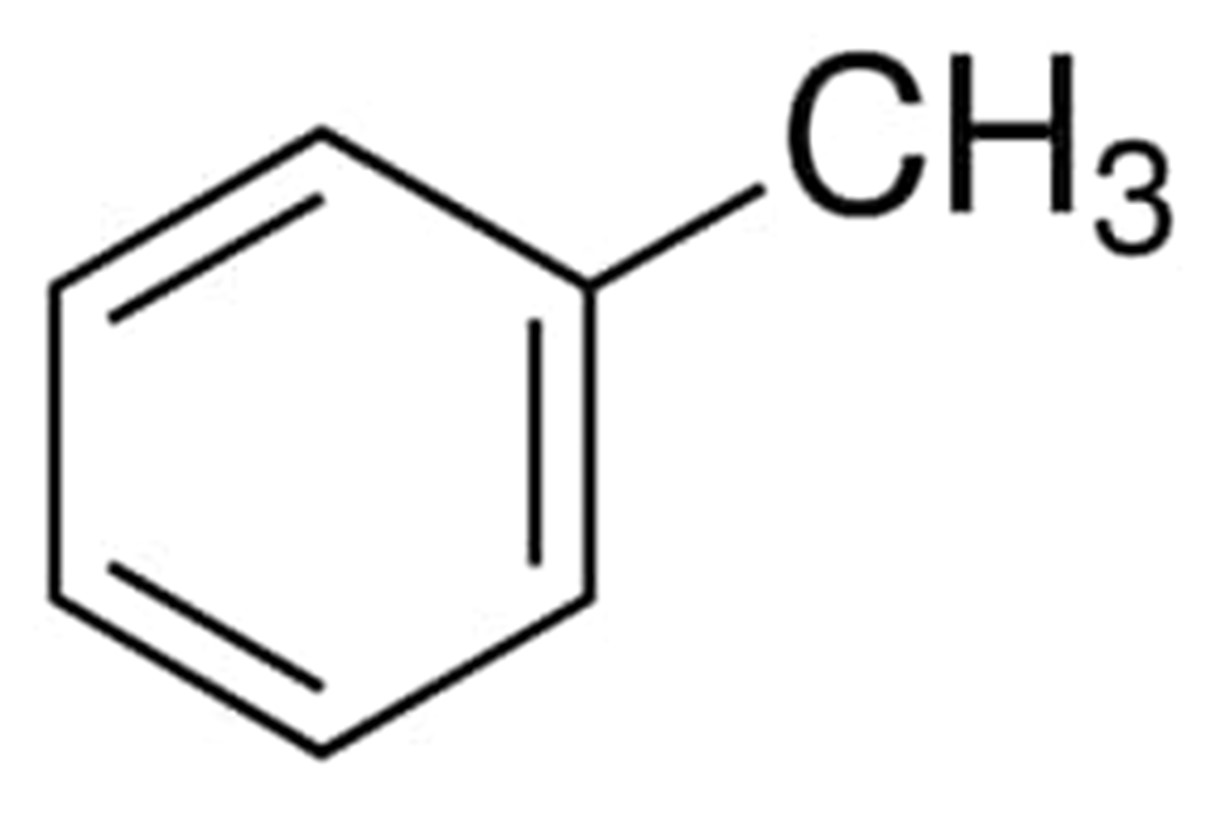
Toluene
To give just one example, the tolyl group is derived from toluene, which is a methyl group-substituted benzene ring in its original form. Never will an aryl group be completely saturated. As a consequence of this, aryl groups must have a structure that is composed of double bonds. Aryl groups are capable of having a wide range of aromatic rings in addition to benzene. The indolyl group is an example of this type of aryl group, and it is found attached to the common amino acid tryptophan. As can be seen up there, the phenyl group originates from the benzene ring.
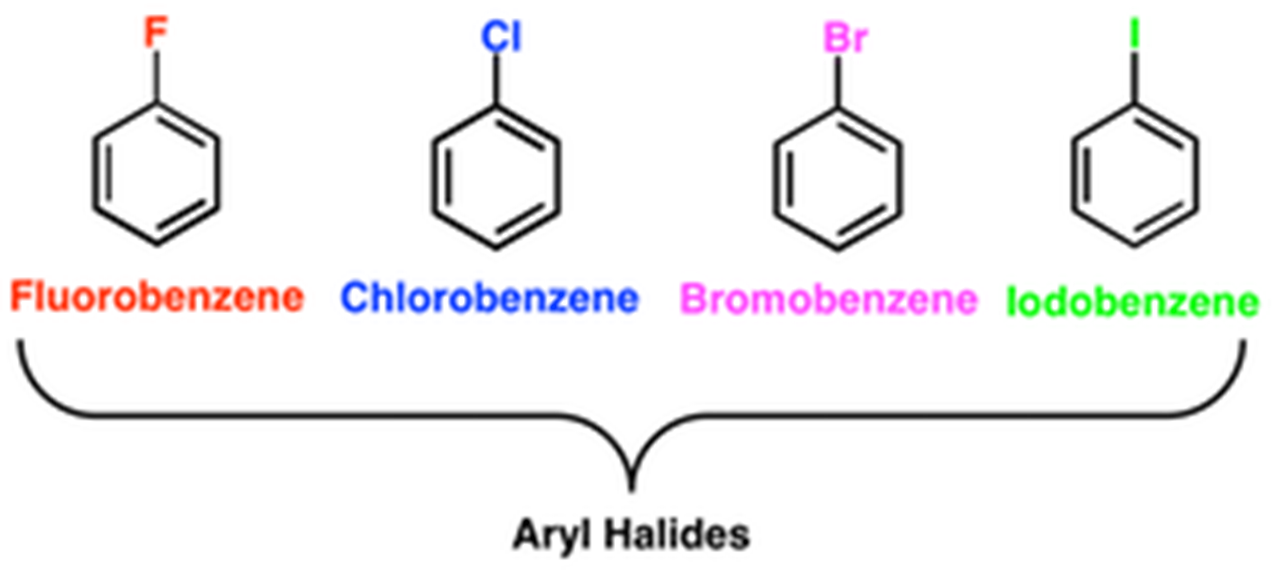
Aryl Halide
A molecule is said to be an aryl halide if the sp2 hybridized carbon of an aromatic ring is directly linked to an atom of halogen in that molecule. Because of the presence of double bonds in the aromatic ring, this structure is unsaturated. There is a possibility that aryl halides will exhibit interactions of a dipole-on-dipole nature. Because of the ring electrons, the bond between carbon and halogen is significantly more stable than the link between alkyl and halogen. The positive charge on the carbon atom is reduced as a result of the aromatic ring providing it with electrons to donate. Electrophilic substitution of aryl halides enables the addition of alkyl groups to the aromatic ring in either the ortho, para, or meta positions. The aromatic ring is open to the possibility of attachment by either one or two halogens. This contains not just the ortho, para, and meta configurations but also the ternary configuration as well. Some of the common aryl halides are Iodobenzene, Chlorobenzene, Bromobenzene and Fluorobenzene which can be written as following chemically:
Uses of compounds having an aryl group
The widespread application of many aromatic chloro compounds as herbicides, pesticides, bactericides and fungicides is nothing out of the ordinary. They have received a lot of attention due to the fact that their negligent usage might have devastating repercussions, which is why they are frequently avoided.