Benzene Compounds: Chemical structure and derived compounds
The most fundamental organic chemical is benzene, which belongs to the group of aromatic hydrocarbons. In crude oil, you may find naturally occurring benzene, as well as many other fundamental petrochemicals. It is an odorless liquid that has a strong resemblance to the smell of gasoline. There is sufficient evidence to support claims that benzene is both carcinogenic and toxic. The production of polystyrene is where it finds the majority of its uses.
Volcanoes and forest fires make benzene, which is also present in many plants and animals; yet, benzene is also a major industrial chemical, created from coal and oil. When it is in its purest form, benzene is a liquid that is clear and has no discernible color. Benzene is utilized extensively in industrial settings for the production of a variety of different chemicals, polymers, detergents, and pesticides. In addition to that, it is also present in gas.
Chemical Structure
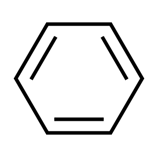
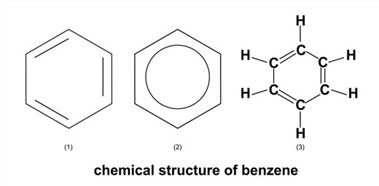
Ever since its discovery, scientists have been interested in learning more about the chemical structure of benzene. Because it has the chemical formula C6H6, benzene is considered to be a cyclic hydrocarbon. This signifies that the carbon atoms in benzene arrange themselves in the shape of a ring that has a total of six members and that each member is attached to a single hydrogen atom. In contrast, the molecular orbital theory states that the formation of the benzene ring is caused by the development of three delocalized - orbitals that span all six carbon atoms. The valence bond theory identifies two stable resonance configurations for the benzene ring, while the molecular orbital theory makes this claim.
Benzene-derived Compounds
Compounds that include benzene rings in their structures are typically referred to as "aromatic compounds" since this is the most common usage of the word "aromatic compound." The name for these compounds comes from the fact that they often have a pleasant and sweet odor (even though benzene itself does not), but the more modern definition of the term has to do with the structure of molecules. Conjugated bonds are cyclic topologies of bonded carbons that contain alternating double and single bonds. These configurations are known as conjugated ring structures.
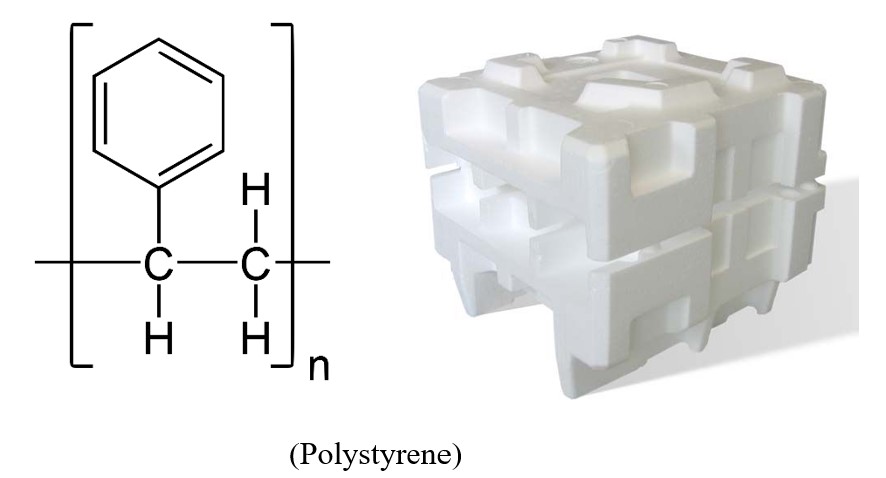
The manufacturing of many helpful compounds that we come into contact with on a regular basis requires the usage of benzene. One noteworthy example is polystyrene, which is produced by the polymerization of styrene. During the polymerization process, a number of individual molecules will react with one another to build larger chains. The many billion kilogrammes of polystyrene that are produced each year are put to use in a wide variety of end applications. Some examples of these uses include food packaging, plastic cutlery, computer housings, foam packing materials and insulation.
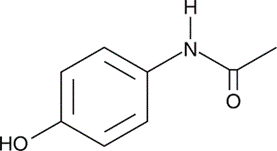
One example of a benzene-based substance that has use in medicine is the analgesic paracetamol, which is also known by its chemical name, acetaminophen. In point of fact, the structure of many pharmaceutical compounds almost certainly includes at least one benzene ring, despite the fact that these compounds' structures are typically far more complicated than the ones shown here. Antibiotics are derived from chemical components, and in a subsequent essay, we will investigate some of these chemical constituents.
Ethylbenzene
Ethyl benzene production accounts for approximately 80 percent of the yearly global output of benzene. In the compound known as ethyl benzene, one of the hydrogen atoms in the ring has been replaced with an ethyl group. In addition to its use in the manufacture of paint, inks, pesticides, and other chemicals, ethyl benzene may also be discovered in coal tar and petroleum. Its applications include the creation of these products. The manufacture of styrene and polystyrene, on the other hand, is where ethyl benzene is most frequently put to use.
Styrene is an essential component in the manufacturing of latex, synthetic rubber, and polystyrene, among other things. Investigate the back of any and all carpets that you could have lying around the house. There is a good chance that styrene-butadiene rubber, which is a kind of styrene, is present in any rubberized backing that you come across. Rubber is utilized not just for flooring but also for the insulation of electrical cables and even the soles of shoes.
Phenol
At some time in our lives, every one of us has likely taken an aspirin to treat a painful condition such as a headache, a bruise, or another complaint. This over-the-counter pain medication is made from phenol, which is a benzene derivative. Phenol is utilized in its creation. In addition, phenol is used as a precursor in the manufacturing of polymers, explosives, and azo dye, which is utilized in a variety of applications, including coloring food and textiles.
In most household cleaners, phenol is present in trace amounts and functions both as an antibacterial and disinfection agent.
Aniline
Aniline is an organic molecule that consists of a phenyl group that is coupled to an amino group. To put it another way, aniline is the most basic type of aromatic amine. It has various applications in industry and is a helpful precursor in the manufacture of fine compounds. In the manufacturing of polyurethane, dyes, and other industrial chemicals, it is largely utilized. It smells like rotting fish, which is a characteristic that other volatile amines share. It is possible to swiftly light it on fire, and as is the case with other aromatic compounds, the flame it creates is a smoky one. Consumption by humans would be extremely hazardous.
In comparison to benzene, it has a greater total number of electrons. As a direct consequence of this, it is able to take part in the electrophilic aromatic substitution processes at a much faster rate. When freshly purified aniline is almost colorless, similar to other oils; however, when it is allowed to be exposed to air, its color gradually changes to yellow or red due to the development of highly pigmented, oxidized impurities.

Toluene
The aromatic hydrocarbon toluene is most commonly referred to by its chemical name, toluol. It is an odorous liquid that is colorless and does not dissolve in water. The viscosity of it is comparable to that of paint thinners. It is a monosubstituted benzene derivative that has a methyl group (CH3) attached to the phenyl ring (Ph). This compound is referred to as methylbenzene in the IUPAC nomenclature. Toluene is frequently used in industry as a feedstock as well as a solvent due to its versatility.
Toluene, a chemical that is used as a solvent in products such as paint thinner, permanent markers, contact cement, and a variety of glues, is occasionally breathed for recreational purposes and has the potential to inflict severe damage to the brain.