Benzoic Acid: Properties, structure, synthesis, applications and safety hazard
Benzoic acid, an organic molecule with the formula C6H5COOH and its molecular structure is composed of carboxyl group that is linked to a benzene ring. This compound is a crystalline, colorless solid at ambient temperature. To be more explicit, "benzoates" refer to C6H5COOH esters and salts.
It was discovered in the early 1600s. Friedrich Wöhler and Justus von Liebig found that benzoic acid has a specific chemical structure. The latter experiments also included research into the relationship between hippuric acid and benzoic acid. Benzoic acid has been routinely used to preserve benzoate-containing cloudberry fruits since Salkowski identified its antifungal properties in 1875.
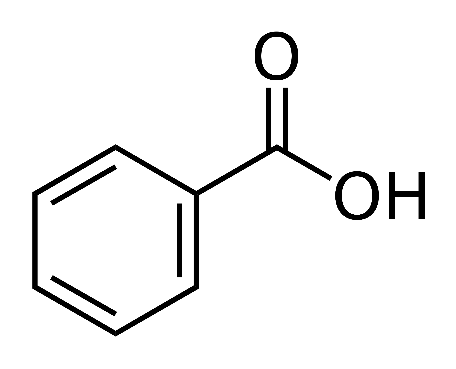
The structure of the C6H5COOH molecule is shown below. Benzene ring with a carboxyl group is attached to this compound. The molecule is made up of 7 atoms of carbon, 6 atoms of hydrogen and 2 atoms of oxygen.
Following diagram depicts the structural formula of benzoic acid:
When benzoic acid solidifies, it takes on a crystalline form that is colorless in appearance and it possesses monoclinic crystal structure as shown in the figure below:
Because of the aromatic ring in its structure, this molecule produces a very faintly pleasant odor and the density of this substance reduces to 1.075 g/cm3 at 130 degrees Celsius.
If we talk about its solubility, it dissolves in water, with solubility of 3.44 grammes per litter at 25 degrees Celsius and 56.31 grammes per litter at 100 degrees Celsius. It is dissolved in alcohol, benzene, acetone and carbon tetrachloride
Synthesis
Industries produce benzoic acid by partially oxidizing toluene with oxygen. Cobalt or manganese naphthenates are catalysts that contribute in this process. The components required for the production of benzoic acid are inexpensive and plentiful, and the procedure yields a high yield.
In the production process, for example, benzotrichloride combines with calcium hydroxide in water. In the procedure, iron or iron salts are utilized as a catalyst. As a result, the calcium benzoate is converted to benzoic acid by hydrochloric acid (HCl). The product may contain a considerable number of chlorinated benzoic acid derivatives.
There is no lack of benzoic acid, and it is inexpensive. Benzoic acid is synthesized in the lab for instructional reasons. It's a regular stage in the preparation process.
To eliminate contaminants from benzoic acid, recrystallization from water is utilized. This is due to the fact that it dissolves readily in warm water but not at all in cold water. This process typically yields 65-70% benzoic acid.
Uses
- Benzoic acid has long been used in topical antifungal medications such as Whitfield's ointment.
- It’s most prevalent use is treating athlete's foot, although it has also been demonstrated to be beneficial in milder instances of ringworm.
- Benzoic acid is a common active ingredient in lipstick and other cosmetics.
- Benzoic acid has a variety of applications other than being a precursor to benzoyl chloride.
- Benzoic acid is found in a variety of facial cleaners, including toothpaste, mouthwash, and face creams.
- Benzoic acid is used to make a range of colors and insecticides.
- Benzoic acid (BA) is frequently used as a preservative in food and drink, particularly carbonated beverages, due to its robust antibacterial effect at lower pH values (2.5-4.0). Bacteria and yeasts, both of which are inhibited by BA, are major causes of food degradation. Drinks containing BA can stay longer without spoiling and reduce nutritional loss, but ingesting too much of it can cause gastrointestinal distress such as diarrhoea and abdominal discomfort, as well as disrupting the body's intermediate metabolic processes. As a result, legislation regulates the maximum permitted levels of BA in all food kinds. The US Food and Drug Administration limits the inclusion of BA to most food categories to 1000 mg kg 1. In China, however, the amount of BA added to carbonated drinks is limited to 200 milligrammes per kilogramme.
Hazards
- The following health outcomes have been associated to benzoic acid exposure, either immediately or shortly after:
- After skin irritation, rashes, redness, and/or a burning feeling may emerge and/or develop.
- Nasal and pulmonary inflammation, which can cause coughing, wheezing, and/or breathing trouble
- Long-term or frequent contact with benzoic acid, on the other hand, can cause skin dryness, cracking, redness, and irritation in addition to the symptoms noticed with brief exposure. In sensitive persons, high levels of benzoic acid may cause skin allergies. If an allergy has established, itching and a rash can occur with very little touch.
When and how to exercise caution
- Stop what you're doing and immediately pour a large bowl of water into your eyes for at least 15 minutes. Please remove your contact lenses before flushing the toilet. Do all you can to receive medical attention.
- The exposed individual must be transported outside as soon as possible to breathe clean air. Contact a doctor as soon as possible.
- Seek medical attention straight away. Drink a glass of milk or water and gargle. At your own risk, induce vomiting.