Bromophenols: types and applications
The term "bromophenol" refers to any type of phenol organobromide that contains one or more bromine atoms in a covalent bond. It is a powder that can range in colour from tan to orange, and it is used as an indicator of acid and base. Bromophenols are the products obtained from the halogenation of phenol by electrophilic bromine.
The chemical structure of a bromophenol compound can be written as following:
Types of Bromophenols
Depending on which carbon atom in the benzene ring of the phenol molecule the bromine atom is attached to, there are a total of 19 distinct types of bromophenols. These bromophenols range in complexity from the simplest mono- to the most complex pentabromophenol.
Monobromophenols
Monobromophenols are found in a total of three isomers, one of which is called 2-bromophenol. This is because a single bromine atom can only occupy one of the three ring positions on a phenol molecule. Pentabromophenol, on the other hand, only exists in a single isomer due to the fact that all five of the phenol ring positions have already been taken up by bromine atoms. In order to synthesize a monobromophenol, only one bromine molecule is incorporated at any carbon of the benzene ring of a phenol compound.
There are many isomeric forms of the Bromophenols which are as following:
- 2-Bromophenol
- 3-Bromophenol
- 4-Bromophenol
All of these isomeric can be discussed in detail as following:
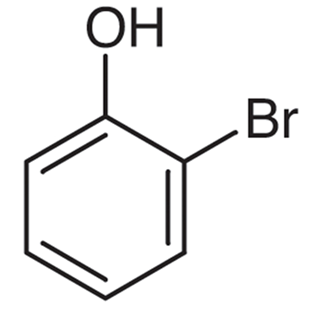
2-Bromophenol
The compound known as 2-bromophenol is an example of a bromophenol. In marine organisms, it functions as a metabolite, which is a useful function. This substance is also known as 2-BROMOPHENOl, 95-56-7, o-Bromophenol, phenol, 2-bromo-, and phenol. Other names for the compound include phenol. According to the collected data, 2-Bromophenol is found in naturally occurring quantities in Ulva lactuca.
The structural formula of a 2-bromophenol compound can be written as following:
The production of organic bromine compounds can be accomplished through a wide variety of different chemical reactions; however, addition and substitution reactions are the ones that are utilised the most frequently in industrial settings.
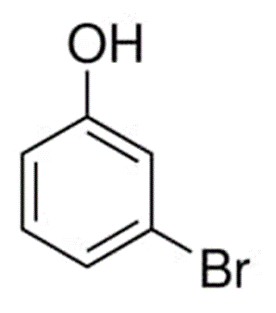
3-Bromophenol
It has a molecular weight of 173.01 and the formula C6H5BrO in its chemical make-up. Its melting point ranges between 28 and 32 degrees, and its boiling point is between 235 and 236 degrees. It is possible to dissolve it in many different solvents, such as water, alkali, ethanol, ether, and chloroform, among others. The solubility of carbon tetra chloride is exceptionally low.
The chemical structure of 3-bromophenol can be represented as following:
3-bromophenol has the ability to inhibit the activity of enzymes. It is possible to make 3-bromophenyl ester from this substance by undergoing a reaction with benzoyl chloride in the presence of triethylamine, which acts as a catalyst. It is impossible to overstate the significance of its role as an intermediate in the production of pharmaceuticals, agrochemicals, and dyestuffs, as well as in organic synthesis, among other processes.
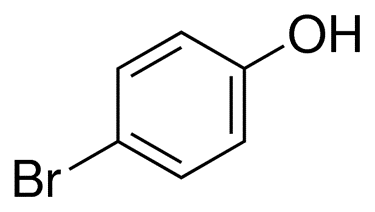
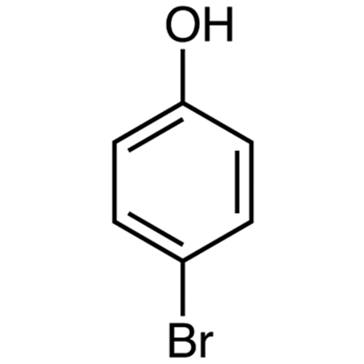
4-Bromophenol
4-Bromophenol, which is a type of bromophenol, is made up of paralyzed pairs of hydroxyl and bromo substituents. In addition to being a metabolite in mice, it is also a POP, a metabolite in the urine of rats, a metabolite in the urine of humans, a metabolite in the urine of marine organisms, and a metabolite in the urine of humans
This molecule has a molecular weight of, and its chemical formula is C6H5BrO. It is easily dissolved in a wide variety of solvents, such as ethanol, chloroform, ether, and glacial acetic acid, amongst many others. There is evidence to suggest that 4-bromophenol is present in naturally occurring quantities in both Bos taurus and Ulva lactuca.
The chemical structure of 4-bromophenol can be written as following:
Uses of Bromophenols
- It plays an important role as a disinfectant in the marine environment.
- Bromophenol blue is used as one of the dyes in various chemical reactions.
- Bromophenol blue has the molecular formula 3H-2,1-benzoxathiole 1,1-dioxide, and its chemical structure consists of two 3,5-dibromo-4-hydroxyphenyl groups in place of the two hydrogens in position 3.
- It can be used as a size marker in agarose gel and polyacrylamide gel electrophoresis, as well as a pH indicator in the laboratory, where it will change colour from yellow to purple depending on the pH level it is exposed to (between 3 and 4.6).
- It has also seen some use in the industrial sector as a dye for various purposes. It can function as a dye in addition to being an acid-base indicator, giving it a high degree of versatility. As a 2,1-benzoxathiole, it is classified as a member of the phenol family, and as an arenesulfonate ester of a sultone, it is placed in the organobromine class.