Carboxylic Acids: Structure, natural sources, properties and applications
Chemical formula for carboxylic is C15H30O4 and its molecular weight is 274.40 g/mol, making it an organic molecule containing a carboxyl functional group. A large number of colorless carboxylic acids are present with an unpleasant goaty smell. Carboxylic acids containing more than ten carbon atoms are waxy solids. As their molar mass increases, their volatility decreases, and their odour fades as a result. When compared to other compounds of the same molecular weight, the boiling points of carboxylic acids are higher.
Some of the main carboxylic acids are as following:
- Acetic acid
- Butyric acid
- Formic acid
- Iso-butyric acid
- Propionic acid
Common names for carboxylic acids come from their source. Formic acid causes ant biting discomfort (Latin word formica, ant) as it was first extracted from red ants. Butyric acid was called after vinegar.
Saturated monocarboxylic acids are alkanoic acids. Names of alkanes with the same number of carbon atoms as the acid. Alkane's "e" is omitted and suffix-oic acid is added. Acetic acid becomes ethanoic acid.
Water is soluble in any carboxylic acid containing one to four carbon atoms. Solubility decreases as molar mass increases.
Carboxylic acids are present naturally and they can also be synthesized. Carboxylic acids are the most important C=O functional groups. Carboxylic acids like citric acid, lactic acid, and fumaric acid are used a lot in the food business. They can be made in a number of ways, including by fermentation.
Carboxylic acids are weak acids because they don't break up completely into H+ cations in a neutral water solution. As a result of this reaction, hydrogen bonds are made between the molecules of acid and water. Because of this, they partly ionize to make H+ and RCOO. They are called "weak acids" because of this. Using the idea of acidity as "something that gives other things protons" (hydrogen ions), what makes carboxylic acids acidic is the hydrogen in the -COOH group.

Structure
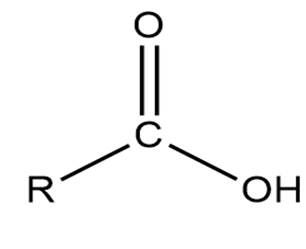
The carboxyl ring and the rest of the molecule are denoted by this group (COOH) in a carboxylic acid's typical formula. R-COOH. With the addition of a hydroxyl group, we have three carbon atoms bonded together in this carboxy group. Carbonyl groups are represented by the R-COOH structure of a carboxylic acid. Carboxylic acid consists of only one hydroxyl group and two covalent bonds.
The following figure represents a carboxylic acid's structure.
Natural sources of Carboxylic acid
Carboxylic acids may be found all over the world. Acids such as lactic, malic and citric acids may be found in fruits such as apples, pears, lemons, oranges and grapefruit, as well as in vinegar.

Properties of Carboxylic Acids
Carbonyl groups are what give carboxylic acid much of its properties. The features of these compounds are discussed in this section in terms of their chemistry and physics.
Physical Properties
- Carboxylic acid molecules are polar because of the presence of two electronegative oxygen atoms. Carbonyl (C=O) and hydroxyl (OH) groups contribute to hydrogen bonding, as do the carbonyl groups.
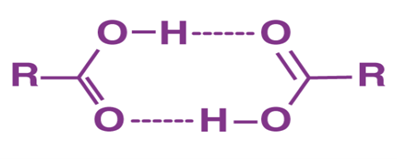
- Dimers of these molecules are formed when the hydroxyl groups of carboxylic acids hydrogen bind together in nonpolar liquids.
- Carbonyl functional group compounds can be dissolved in water at a rate that is directly related to their size. The smaller a chemical is, the more solubility it possesses (the shorter the R-group).
- Acids with a higher boiling point than water are called carboxylic acids.
- Because of their ability to donate protons, these acids are referred to as Bronsted-Lowry acids.
- Generally speaking, they have a distinct vinegar scent. Despite this, their esters are valuable in perfumery because of the pleasant fragrances they emit.
Chemical Properties of Carboxylic Acids
- The Hell-Volhard-Zelinsky chemical reaction may readily halogenate a carboxylic acid with a -carbon.
- The Schmidt reaction can be used to convert carboxylic acids into amines.
- Hydrogen can be used to initiate a hydrogenation process to decrease carboxylic acid to alcohol.
- Esters are generated when alcohols combine with carboxylic acids.
Uses of Carboxylic Acids
- Polymers, adhesives and prescription drugs all benefit from the use of these acids and their derivatives. They may be used to make a wide range of products, including solvents, antimicrobials, food additives, and flavors, among other things.
- The usage of carboxylic acids in a variety of food and beverage items is also commonplace.
- Carbonic acid is also utilized in the textiles, leather and rubber industries.
- Cationic acid, ethylene-diamine-tetra acetic, was utilized as a chelating agent.
- These compounds are used in the manufacturing of a wide range of medications. Carbamic acids therefore play a crucial role in the pharmaceutical business.
- Compounds with carboxyl functional groups are used in a wide range of polymer production processes.
- In many cases, the smell of carboxylic acids can be noxious. Carboxylic acid esters are frequently used in the fragrance business because of their fruity and pleasant aromas.