Chloromethyl: compounds, synthesis and safety
Chloromethyl
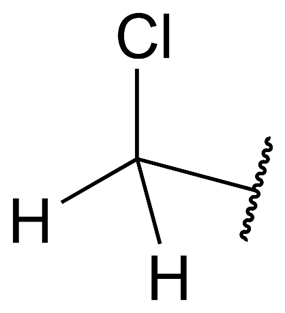
In the field of organic chemistry, a functional group known as the chloromethyl group (CH2Cl) can be found. In the formula for the methyl group (CH3), one hydrogen atom was replaced with a chlorine atom, which resulted in the creation of a new chemical entity that was subsequently given a new name. Compounds like chlorobenzene are part of the larger category of organochlorines, which is represented by this structural class as a subset.
A chloromethyl group can be represented structurally as follows:
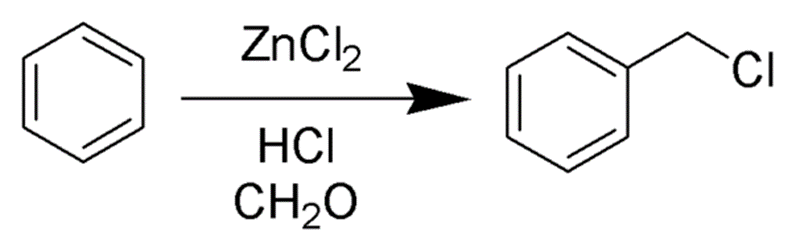
Chloromethylation
The introduction of a chloromethyl group into aromatic compounds is accomplished through the process known as chloromethylation, which is facilitated by the Blanc reaction. The process of chloromethylation can be represented as following:
Trichloromethyl group** **
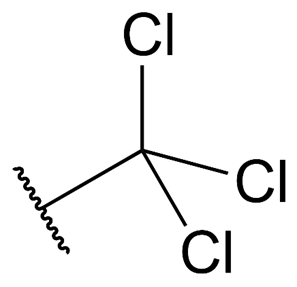
The chemical symbol for trichloromethyl, also known as TCM, is -CCl3, and it denotes a functional group. By exchanging each hydrogen atom in the methyl group for a chlorine atom, the name "is group" was derived from the name of the methyl group (which has the formula -CH3). This resulted in the methyl group having the formula -CH3. The organochlorines can be thought of as a larger group, of which this structural group is a subset. Compounds such as trichloromethane H-CCl3, 1,1,1-trichloroethane H3C-CCl3, and chloral HOC-CCl4 are typical examples of the substances that fall into this category.
The trichloromethyl group has a high electronegativity because it contains three chlorine atoms. Because of this, trichloromethyl-substituted acids, such as trichloromethanesulfonic acid, are typically more powerful than their counterparts that do not contain this substitution. For example, because of its low pKa value of 3.6, trichloroacetic acid (HOOC-CCl) is considered to be a weak acid.
A trichloromethyl group can be written as following:
The pKa of the number 3 is only 0.77, which is significantly lower than the pKa of acetic acid, which is 4.76. It is well known that trichloromethyl groups, similar to those that can be found in trichloroethanol, can lower the basicity of organic compounds using the same mechanism.
Chloromethyl Methyl Ether
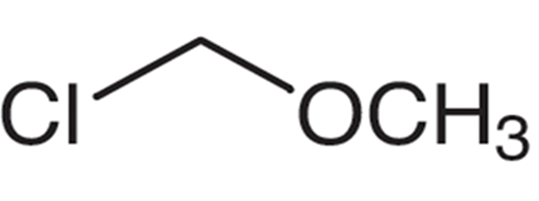
Chloromethyl methyl ether, also known as CMME, is a chemical compound that can be represented by the formula CH3OCH2Cl. It is a chloroalkyl ether and it is a liquid that has no discernible colour. It plays an essential role in the production of alkylating agents. Because of its application in the process of organic synthesis, it is frequently referred to as MOM-Cl or MOM chloride. This is due to the fact that it is used to introduce the methoxymethyl (MOM) protecting group. It is utilised in the capacity of a chloromethylating agent in certain iterations of the Blanc chloromethylation.
A chloromethyl methyl ether group can be written as following:
Synthesis
A Lewis acid catalyst is utilised in the reaction between dimethoxymethane and acetyl chloride, which results in the production of chloromethyl methyl ether in situ. Using this method, one can obtain a solution of chloromethyl methyl ether in methyl acetate that has a high degree of purity. The only detectable impurity is dimethoxymethane, which can be avoided by using an acyl chloride with a high boiling point. This method can also be used to achieve purity. In contrast, the classical procedure involving formaldehyde, methanol, and hydrochloric acid (FMAH-HCl), which is described in Organic Syntheses, results in the production of a highly contaminated bis(chloromethyl) ether product that needs to be fractionally distilled.
Safety
The amount of time it takes for three distinct types of standard aqueous quench solutions (ammonium chloride solution, water, and sodium carbonate solution) to eradicate any and all traces of chloromethyl methyl ether has been timed by researchers. After 15 minutes of vigorous stirring with the quench solution, the chloromethyl methyl ether solution in toluene/methyl acetate was completely degraded. This was achieved by removing all of the chloromethyl methyl ether (below the detection limit).
The link between CMME and cancer in humans has been established by scientific research. Long-term exposure has been linked to the development of small cell carcinoma, one of the subtypes of lung cancer. Despite the fact that there is no known safe exposure level for this chemical at the present time, the Occupational Safety and Health Administration (OSHA) continues to regulate it along with 12 others.
Facilities that produce, store, or use significant amounts of this substance are required to file detailed reports detailing their activities with this substance in accordance with Section 302 of the United States Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002). These facilities are required to submit these reports every three years. The Prohibition of Certain Toxic Substances Regulations in Canada include a schedule called "1."