Chloropyridine: Common isomorphs, synthesis, reactions and applications
Chloropyridine is the chlorine derivative of pyridine with a molecular formula C5H4ClN and molecular weight 113.54 g/mol. It is also referred as an Organohalide or a Halopyridine molecule and is basically used as an intermediate in many chemical reactions.
The development of pharmaceuticals, agrochemicals, and metal complexes is being hampered by a lack of methods that can selectively halogenate the C–H precursors of pyridine C–H ligands. In order to install heterocyclic phosphines at the 4-position of pyridine as phosphonium salts and then replace them with halide nucleophiles, a process known as "phosphonium salt installation" has been introduced.
This strategy is effective for the late-stage halogenation of complicated pharmaceuticals, and it may be used to a wide variety of unactivated pyridines. The removal of phosphine has been proven to be the stage in the creation of C–halogen bonds that determines the rate at which the bonds are formed through computer research. The reactivity of 2- and 3-substituted pyridines is different from that of the parent molecule. This is because steric interactions take place during the breaking of the C–P bond.
There are three main isomeric forms of chloropyridine which are as following:
- 2-Chloropyridine
- 3-Chloropyridine
- 4-Chloropyridine
All of these isomers of chloropyridine can be discussed in detail as following:
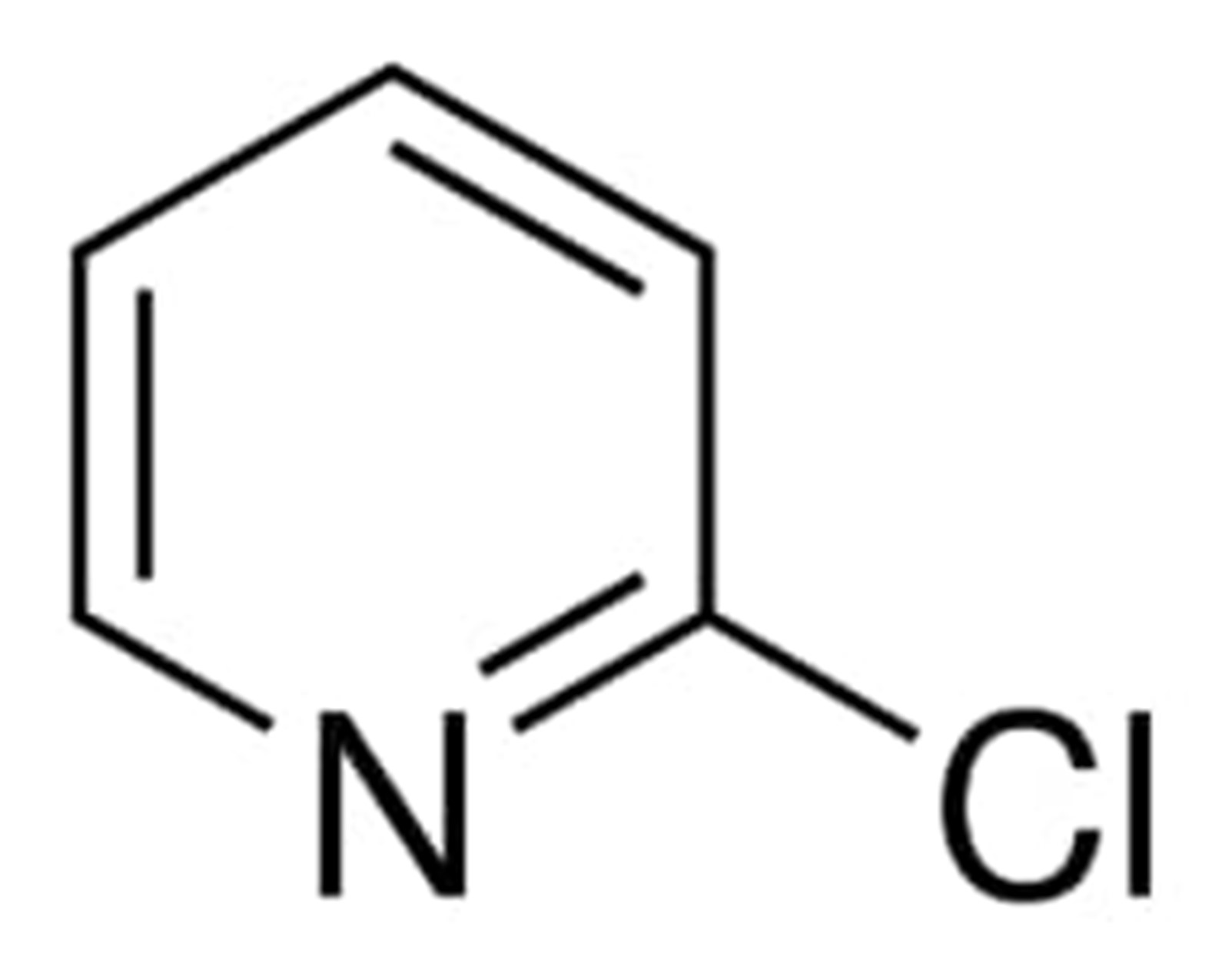
2-Chloropyridine
2-Chloropyridine is a halogenated derivative of pyridine with the chemical formula C5H4ClN and the molecular weight 113.54 g/mol. Its IUPAC name is 2-chloropyridine and is also known by the names such as 109-09-1, O-Chloropyridine, Pyridine, 2-chloro- and Alpha-Chloropyridine.
In the agricultural business, it is mostly used to produce fungicides and insecticides. In addition, it is used to produce antihistamines and antiarrythmics for medicinal use.
Following diagram shows the structural formula of 2-Chloropyridine
Synthesis of 2-Chloropyridine
2-Choropyridine is made by combining pyridine with chlorine in a single process. When 2-chloropyridine is first created, it undergoes additional reactions to form 2, 6-dichloropyridine.
Alternatively, pyridine-N-oxides can be used to easily manufacture 2-chloropyridines in high yield.
When 2-hydroxypyridine was first synthesized, it was by chlorinating it with phosphoryl chloride, yielding 2-chloropyridine.

Reactions of 2-Chloropyridine
When nucleophiles are added to 2-Chloropyridine, the heterocycle is transformed into pyridine derivatives with the second and fourth carbons replaced. In order to separate the desired isomer from a mixture of compounds produced by 2-chloropyridine reactions, further workup is usually required.
Uses of 2-Chloropyridine
Disopyramide and chlorphenamine are examples of commercially available pyrithiones and chlorphenamines. Chloride is displaced during these transformations. Shampoos containing 2-mercaptopyridine-N-conjugate oxide's base, pyrithione, include a fungicide known as pyrithione. 2-chloropyridine-N-oxide is the product of the oxidation of 2-chloropyridine. In the presence of a base, phenylacetonitrile and 2-chloropyridine can be combined to produce pheniramine, an antihistamine.
Synthesis of methylphenidate, an ester of 2-phenyl-2-piperidilacetic acid, is as follows. To get acetonitrile, you need to combine 2-chloropyridine with an acid and benzylcyanide. Nitric acid hydrolysis and subsequent esterification with methanol yields the methyl ester of -phenyl— nitriles (2-pyridylacetic acid). Hydrogen over platinum reduces the pyridine molecule into a piperidine, resulting in methylphenidate.
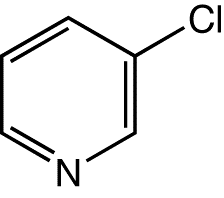
3-Chloropyridine
3-Chloropyridine is classified as an organohalide and may be represented chemically by the formula C5H4ClN. It is a colorless liquid that is used almost exclusively in the construction of organic compounds during the process of chemical synthesis. One of the many different kinds of coupling reactions that may be carried out on the molecule is known as the Heck reaction.
The boiling point of 3-chloropyridine is148 °C (298 °F; 421 K), its density is 1.194 g/cm3 the refractive index of this compound is appeared to be 1.533.
Following diagram depicts the structural formula of 3-Chloropyridine
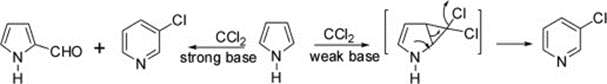
Synthesis of 3-Chloropyridine
Carbenes are an electrophile because they lack electrons. This is how 3-chloropyridine is formed by reacting pyrrole with dichlorocarbene, a weak base, to produce dichlorocyclopropane as an intermediate, followed by ring expansion. Carbene, on the other hand, created a strong base and a combination of pyrrole-2-carboxaldehyde and 3-chloropyridine when it was used in the process.
Uses of 3-Chloropyridine
As a pharmaceutical intermediary, 3-Chloropyridine can be found in a variety of products. 3chloro-pyridine-1-oxide may be made with this compound.
Storage
This chemical must be kept in a cold, dark area and in a well-sealed container. Oxidizing agents are incompatible.
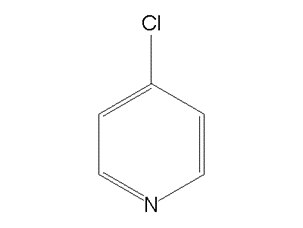
4-Chloropyridine
4-chloorpyridine is a halopyridine with the same molecular formula and molecular weight as of above two isomers. It is also colorless in appearance and is known by the names such as 4-CHLOROPYRIDINE, 626-61-9, Pyridine, 4-chloro-, 4-chloro-pyridine and EINECS 210-956-2
It is used as a catalyst in the one-pot pyrrolation/cyclization of anthranilic acids for the manufacture of fluorazone derivatives. When 4-chloropyridine and mercaptoacetic acid are combined, the result is pyridylmercaptoacetic acid, which serves as a precursor to the cephalosporins.
Following diagram depicts the structural formula of 4-Chloropyridine:
Synthesis of 4-Chloropyriidne
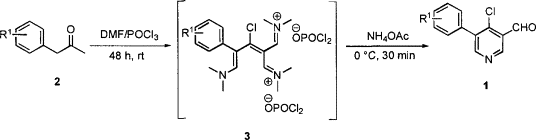
Carbonyl compounds with two enolizable carbons next to the carbonyl have been used to synthesize 4 4-chloropyridines. Vilsmeier-Haack reaction conditions were used on ketone 2 to produce conjugated iminium salts, which, following basic workup, reacted with ammonium acetate to make 4-chloropyridines.
Uses
Pyridylmercaptoacetic acid, which is a precursor to cephalosporin antibiotics, is formed when mercaptoacetic acid interacts with 4-chloropyridine (e.g., cephapirin sodium salt).
Environmental properties of Chloropyridine
Even though pyridine is a good source of carbon and nitrogen for some microbes, the addition of a halogen moiety considerably slows the decomposition of the pyridine ring. The chloropyridines, except for 4-chloropyridine, were shown to be resistant to microbial degradation in soil or liquid media. The total deterioration period was estimated to be greater than 30 days. Volatilization losses of 2-Chloropyridine from water are considerable, but less so when it is present in soil.
Uses of Chloropyridine
The first nonbenzodiazepine with a pharmacological profile equivalent to chlordiazepoxide and nitrazepam is Zopiclone, which is made from 2-amino-5-chloropyridine and utilised as a CNS medication. Insomnia is addressed with the use of zopiclone.
Linopirdine is an oral cognitive enhancer that stimulates the release of CNS neurotransmitters to alleviate deficits associated with Alzheimer's disease (such as acetylcholine, dopamine, and serotonin). Under phase transfer conditions, two equivalents of 4-picolyl chloride hydrochloride are used to alkylate 1-phenyloxindole. This cognitive enhancer's purpose is to make up for these shortfalls.