Glacial Acetic Acid: synthesis, applications and safety hazards
Anhydrous acetic acid, also known as glacial acetic acid, is a form of acetic acid that does not contain any water at all. It is an organic compound that can be written as CH3COOH. Vinegar is the term given to a solution of acetic acid that has been diluted with water. This solution is also known as ethanoic acid or ethylic acid. When compared to other acids, this one is quite weak.
The phrase "anhydrous acetic acid" refers to acetic acid that has such a low water content that it is nearly devoid of any traces of water at all (less than 1 percent). It is referred to as glacial because it is able to freeze into ice-like crystals of solid acetic acid at a temperature that is just slightly below room temperature, namely 16.7 degrees Celsius. The melting point of acetic acid drops by 0.2 degrees Celsius for every 0.2 grammes of water that is evaporated.

The majority of people refer to acetic acid with a chemical term ethanolic acid (CH3COOH). It exudes a strong odour and possesses a harsh flavour that can be immediately identified as the aroma and taste of vinegar due to the organic nature of its composition. Vinegar typically contains between 3 and 9 percent acetic acid on average. It is a carboxylic acid and after formic acid, it has the second-lowest complexity of any carboxylic acid.
The chemical structure of acetic acid can be written as following:
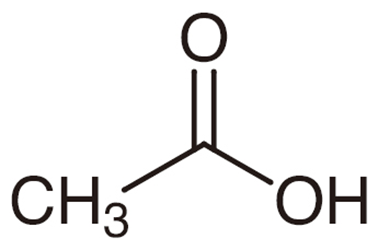
The production of vinegar and the chemical reactions that lead to the creation of cellulose acetate and polyvinyl acetate are the two most prevalent applications for acetic acid. Acetic acid, which is included in E260, is employed both as a flavouring ingredient and as a means of ensuring that foods maintain the appropriate amount of acidity. In the field of the chemical sciences, it is also highly significant to use as a reagent.
Synthesis
On a yearly basis, the world's population consumes around 6.5 metric tonnes of acetic acid, yet recycling efforts only yield approximately 1.5 metric tonnes of the substance. The production of acetic acid almost always involves the utilization of petrochemical feedstock. When progressively diluted with acetic acid solution, the solid form of acetic acid known as a "stalactite" can be utilised to produce glacial acetic acid. This process is referred to as "stalactite melting." Because of this, an ice stalactite is produced as a result (which could be considered to be frozen). At the same time as the liquid is washing away the impurities, the pure acetic acid is sticking to the frozen surface. An analogy for this phenomena is the capacity of a water glacier to maintain the purity of its water despite the fact that it floats in the middle of the ocean.
Uses
The following are some of the most common applications for glacial acetic acid:
- As a key input, glacial acetic acid is necessary for the production of synthetic fibres such as cellulose acetate and rayon.
- Acetic acid is used in the production of a wide variety of personal care products, including fragrances, acetone, and esters, to name just a few of these products.
- Glacial acetic acid is used in a variety of industries, including as a solvent for photographic film, in the production of dyes and inks, in the conversion of latex into rubber, in the production of polyvinyl acetate for use in wood glue, in the production of substrates, in the production of pesticides, in the production of white lead, and in the production of plastic bottles for soft drinks. Glacial acetic acid also has many other applications. Glacial acetic acid is put to use for a variety of critical purposes in addition to being utilised in the production of white lead and pesticides.
- As a chemical reagent, glacial acetic acid is frequently used in the world of higher education. Students' attention is drawn to the acid because of its propensity to draw water from its surroundings and its ability to demonstrate its acidic properties by turning blue litmus paper into red. In addition to the salt, the reaction results in the production of a very high concentration of carbon dioxide when sodium bicarbonate is introduced into it.
- Glacial acetic acid is frequently utilised in clinical laboratories for the purpose of conducting blood analysis.
- In order to preserve pickles and other vegetables during the canning process, a glacial acetic acid derivative that has been diluted with water is utilised. In the industrial cleaning industry, this chemical is also utilised for the purpose of scale removal. In addition to its more typical applications in cleaning and canning, diluted glacial acetic acid can be used to treat or prevent outer ear infections caused by bacteria or fungus. This can be done by either removing the infection or preventing it from occurring in the first place.
Hazards
Glacial acetic acid is corrosive and can cause skin irritation if it comes into touch with the skin. On the other hand, acetic acid, which is the acid that is present in vinegar, is regarded to be mild and safe for human consumption. Damage to tissues can be caused by glacial acetic acid, and solutions that are extremely concentrated (more than 80% acid) can produce burns ranging from moderate to severe. When exposed to acetic acid vapors, the upper respiratory system, the eyes, the skin, and the mucous membranes might all feel irritation.