Hydroxypyridine: Common isomorphs, synthesis, applications and storage
Hydroxypridine is an organic compound having a hydroxyl group attached to the pyridine molecule. The IUPAC name of hydroxypyridine is Pyridin-2(1H)-one, whereas the chemical formula for these compounds is C5H4NH(O). It is a compound that is solid in nature and has no color. This chemical is well-known for its capacity to manufacture tautomers, in addition to the hydrogen-bonded dimers and tautomers that it can already produce.
Hydroxypyridines are zwitterions because they have properties of both weak acids and weak bases. Due to the fact that their canonical forms are charge-free, the zwitterions of 2- and 4-hydroxypyridine are referred to as 2- and 4-pyridones.
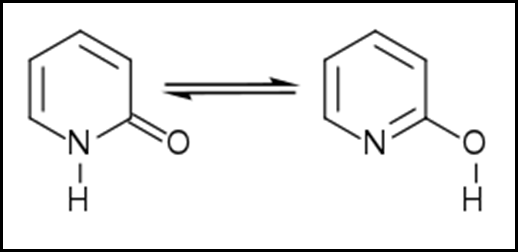
In aqueous solution, hydroxypyridines are most commonly found in either the oxo or pyridone form, depending on the specific compound. With the exception of 3-hydroxyisoquinoline, the equilibrium leans more heavily toward the benzopyridone form for - and -hydroxybenzopyridines and -benzazines.
The hydroxypyridine molecule has the following chemical structure:
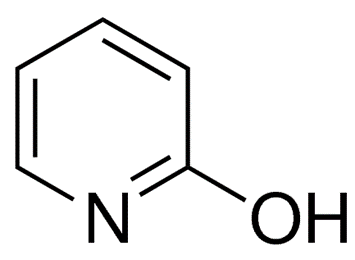
In aqueous solutions, hydroxy and zwitterionic -hydroxypyridines coexist in amounts that are comparable to one another. The effects of 3-Hydroxypyridine are quite similar to those of phenol in many respects. It is a salt that can be alkylated by alkyl and produces a vibrant violet color when coupled with ferric chloride and sodium hydroxide. This salt can also be alkylated by alkyl.
Tautomers are chemical compounds that may easily change into one another, and hydroxypyridines can be found in the form of a tautomer. The majority of the time, the rearrangement of the hydrogen atoms in a compound is what causes the tautomerism that occurs in the complex. The process of tautomerization is referred to as the phenomenon of tautomerism, which is also referred to as desmotropism. Tautomerization may be seen, for example, in amino acids and nucleic acids, which are two of the most fundamental building blocks of life.
Synthesis
Hydroxypyridines can be produced as a byproduct of a cyclization reaction. These hydroxypyridines can subsequently be converted to 2-pyridone by an ammonia exchange reaction.
According to the available research, hydroxypyridine exist as one of two major isomers.
- 2-Hydroxypyridine
- 3-Hydroxypyridine
It is feasible to discuss the isomeric forms of hydroxypyridine in the following manner:
2-Hydroxypyridine
The white to grey to brown powder to crystal solid, 2-Hydroxypyridine (also known as 2-Pyridinol, 2-Pyridone, or 142-08-5) is extremely water-soluble and also soluble in methanol, and ethanol. However, it is insoluble in benzene and ether. The crystal solid form of 2-Hydroxypyridine may be found in all three colors.
Uses
2-Hydroxypyridine, which is widely considered to be one of the most adaptable catalysts in the industry, may be utilized in a variety of acylation procedures in addition to the aminolysis of a polyglutamate. It is essential in the production of peptides.
Storage
It is recommended that 2-Hydroxypyridine be stored in a dark area so that it is not exposed to direct sunlight.
3-Hydroxypyridine
For 3-Hydroxypyridine, also known as pyridinol, 109-00-2, or 3-Pyridinol, the hydrogen atom in position 3 has been switched out for a hydroxy group, transforming it into monohydroxypyridine. Other names for this compound include pyridinol. It may be found in a powder form that is either white or brown, and it has a melting point of 126 degrees Celsius, a boiling point of 180 degrees Celsius/22 mmHg, and a solubility in water of 33 grammes per litre when the temperature is 20 degrees Celsius.
Because of its solubility, 3-hydroxypyridine is easier to distribute in the environment, which can lead to considerable damage to the natural world. It has been demonstrated that the hallucinogenic plant Salvia divinorum, which is native to Mexico, produces it as a thermal breakdown product in its smoke.
Synthesis
Due to the high costs associated with its production, the molecule 3-hydroxypyridine, which is both a desirable and useful chemical intermediate, cannot be employed in commercial applications. According to the available research, a number of different attempts have been taken to solve the problems that result in higher production costs.
In order to produce 3-hydroxypyridine, one tried-and-true method involves heating the sodium and potassium salts of 3-pyridinesulfonic acid to very high temperatures and fusing them together. Because it has been demonstrated to be practically difficult to produce regular quantities of high-quality 3-hydroxypyridine, this strategy has only a limited amount of promise for use in commercial settings.
Another method for the production of 3-hydroxypyridine involves the use of ammonium salts of 3-pyridinesulphonic acid in conjunction with potassium hydroxide as a fusion catalyst (3-HP). This strategy cannot be used in a plant because it produces foaming as a result of the release of ammonia during the reaction in question. Because of the frothy reaction mixture's contribution, maintaining consistent heating becomes increasingly challenging as the batch size grows.
Uses
The chemical 3-Hydroxypyridine is used as a starting point in the production of a number of different chemicals, including the insecticide trifloxysulfuron and the antihistamine pirbuterol.