Iodopyridine: Common isomorphs, synthesis, side effects and applications
Iodopyridine is one of a large variety of commercially accessible, highly halogenated heterocyclic compounds. Several of these highly halogenated heterocyclic derivatives are routinely synthesized on an industrial scale for use in biological applications and as intermediates. Poly substituted heterocyclic system syntheses are becoming increasingly important in the fields of life sciences and pharmaceuticals during the discovery phase. The utilization of pyridine derivatives as valuable building blocks in the production of multifunctional pyridine derivatives may also be of benefit.
There are three main isomeric forms of Iodopyridine which are as following:
- 2-Iodopyridine
- 3-Iodopyridine
- 4-Iodopyridine
These isomers of Iodopyridine can be discussed in detail as following:
2-Iodopyridine
2-iodopyridine is a halopyridine with a chemical formula C5H4IN and the molecular weight 205.00 g/mol. In order to produce 2-iodopyrdine, pyridine is iodized at the carbon number 2 position. It is known by the names such as 2-Iodo-pyridine, Pyridine, iodo- Iodopyridine and 5029-67-4.
The boiling point of this compound is 52.0 degrees Celsius (0.9 mmHg) and its density is 1.9280 grammes per millilitre.
In the production of 2-iodopyridine, iodotrimethylsilicone is utilized as a catalyst. This may be done using either 2-chloropyridine or 2-bromopyridine.
The chemical make-up of 3-iodopyridine is depicted in the following figure:
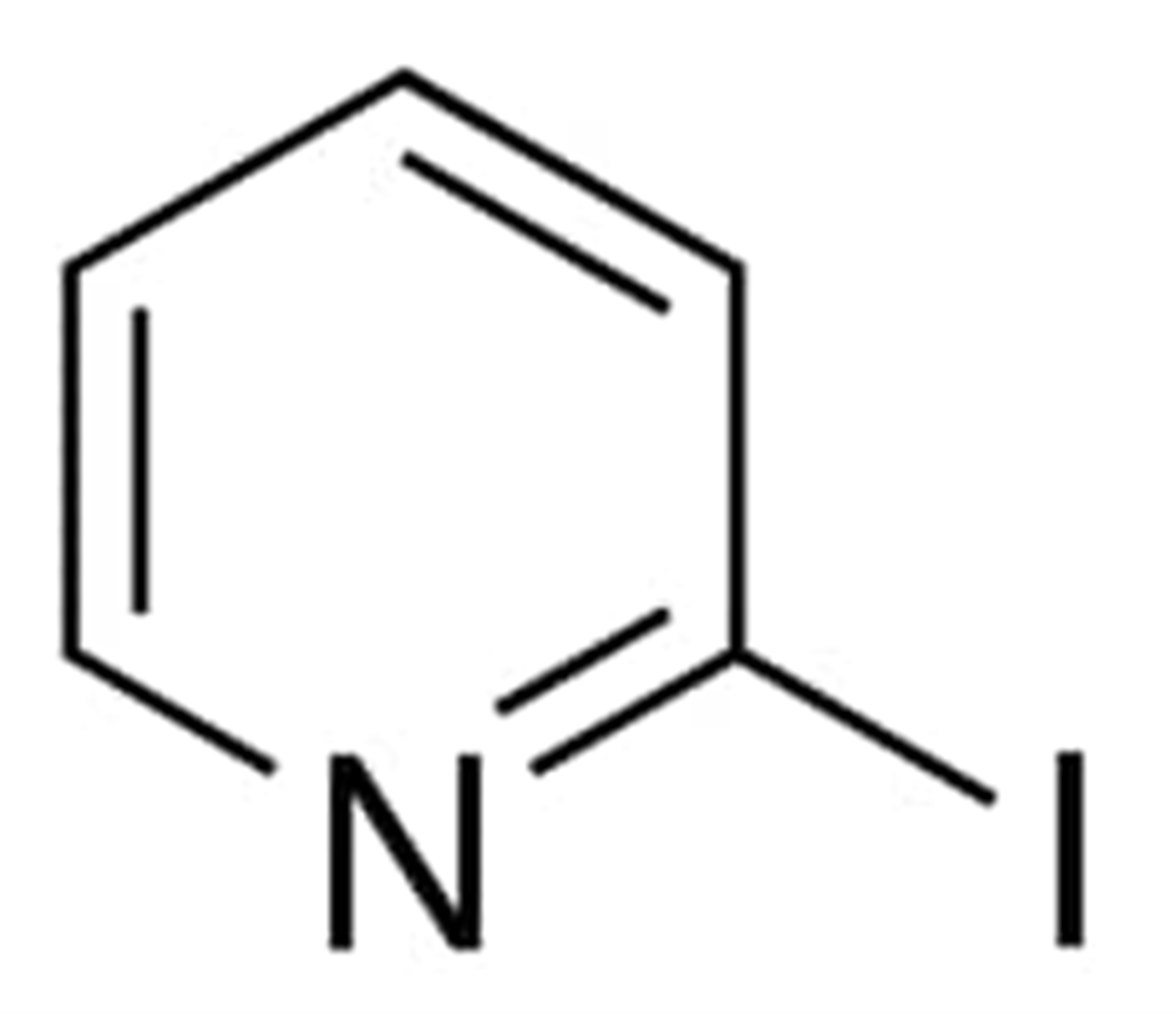
Uses
2-iodopyridine is a reagent that is frequently used in the production of human NAD+-dependent, 15-hydroxyprostaglandin dehydrogenase inhibitors.
3-Iodopyridine
Pyridine is halogenated with iodine at carbon number three to make 3-iodopyridine. Its molecular formula is C5H4IN with a molecular weight 205 g/mol. According to the CAS registry, it has the registration number as 1120-90-7.
The IUPAC name of this compound is 3-iodopyridine and is also known by the names such as 3-iodo pyridine, 3-iodo-pyridine, Pyridine, 3-iodo, 3-iodpyridin, 3-iodopyridine, pubchem6634, Acmc-1bvap and ksc490s0t.
The temperature between 53 and 56 degrees Celsius is where the 3-iodopyridine reaches its melting point and the ignition temperature of 3-iodopyridine is 106 degrees Celsius.
The chemical composition of 3-iodopyridine is depicted in the following figure in structural form:
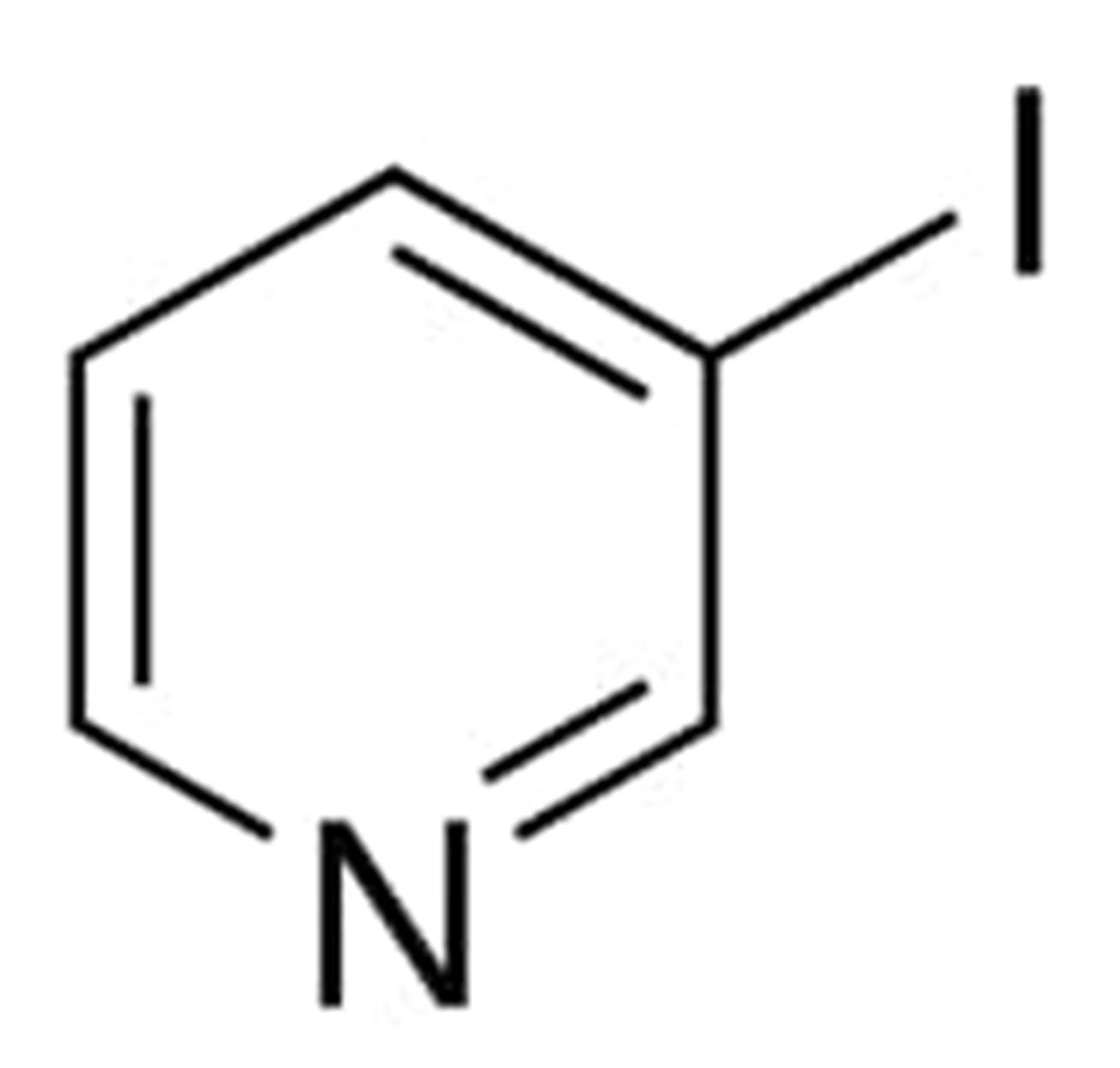
Synthesis
The synthesis of 3-iodopyridine is made possible by attaching a functional group containing iodine to the third carbon position of the pyridine ring.
Uses
The synthesis of pyridine alkaloids, such as theonelladins C, niphatesine C, xestamine D and theonelladins D, is possible with the help of 3-iodopyridine.
Phenazopyridine is an azo dye that is used in the treatment of urinary tract infections. 3-Phenylphenazopyridine is an impurity of Phenazopyridine, and 3-Iodopyridine-2,6-diamine is an intermediary in the manufacture of 3-Phenylphenazopyridine, utilized for the treatment of pain (urinary tract).
4-Iodopyridine
4-iodopyridine is a dark brown to brownish-green colour compound with the same chemical formula and molecular weight as the above two. In order to get 4-iodopyridine, you must bind to the pyridine ring's carbon number 4 an iodine functional group.
The chemical make-up of 3-iodopyridine is depicted in the following figure as a structural formula.
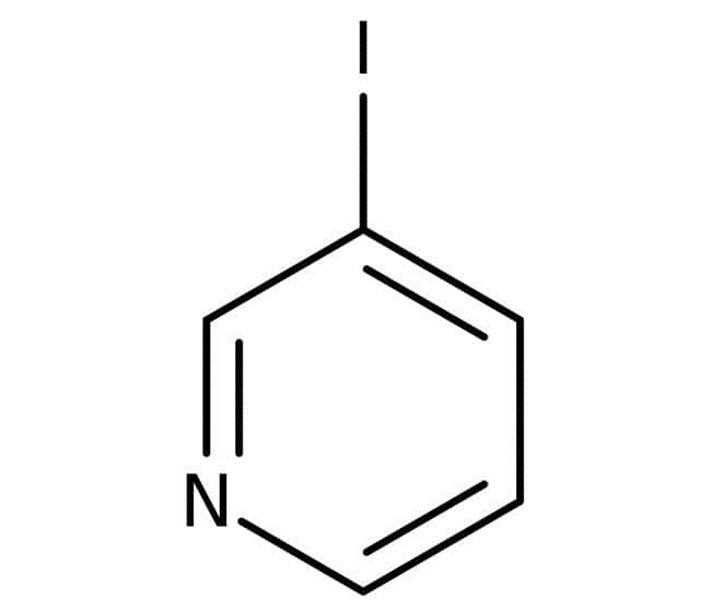
The melting point of 4-iodopyridine falls somewhere in the region of 92.0 to 96.0 degrees Celsius. The 4-iodopyridine is soluble in water, however its solubility in water is on the lower end of the scale for the spectrum of substances that are soluble in water that is slightly soluble. Some of the other names that are used to refer to 4-iodopyridine are 4-iodopyridine N-oxide and 49836-05-7.
Synthesis
It is feasible to manufacture large yields of 4-iodopyridine through the utilisation of a diazotation-Sandmeyer reaction carried out at a low temperature and utilising the right amine. Effectively transforming this chemical into the air-stable pinacol ester of 4-pyridylboronic acid is one of the potential downstream outcomes of interacting with it.
Uses of Iodopyridines
Halogenated heterocyclic compounds, such as pyridine, are characterized by the presence of one or more halogen atoms, which can be bromine, chlorine, fluorine, or iodine. In the field of synthetic chemistry, halogenated pyridine compounds are noteworthy due to the fact that these compounds are utilized as vehicles in the synthesis of other organic molecules. In order to exert control over their functions, their structures can be altered by heterocyclic processes. When it comes to synthetic reactions, organo chlorine and iodine compounds are some of the most commonly employed groups. These reactions are used to produce compounds like 2-iodopyridine, 3-iodopyridine, and chloropyridine, amongst others.
Side effects
- Toxic if swallowed, may cause serious problem if taken in orally.
- Harmful in contact with skin. When comes in contact with skin can cause severe irritation and even causes corrosion.
- If comes in contact with eyes can severe irritation and can cause serious damage.
- If inhaled can cause lungs damage and can affects the respiratory tracts in a single expose.
Storage
This white powder should be stored bellow 30 °C