Ketones: Nomenclature, classification, biochemical significance, applications and toxicity
Ketones are a group of organic compounds that have a carbonyl group that is a link between oxygen and carbon. The remaining pair of bonds are to carbon or hydrocarbon radicals (R):
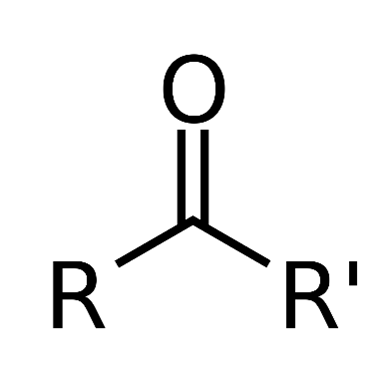
From a biological point of view, molecules of ketone are very important. They are present in sugars and pharmaceutical chemicals, like steroid hormones. Each cortisone molecule is composed of three ketone groups.
Ketones aren't made in large quantities in factories. They are almost perfect chemical intermediates because they can be made in many different ways, are relatively stable, and react strongly. Ketones are used as building blocks in the synthesis of many different types of complex chemical molecules. Most of the time they have uses in manufacturing of lacquers, explosives, textiles and paints. Ketones can be used for many things, like tanning and preserving food.
When most people think of ketones, they think of acetone (CH3COCH3), a sweet-smelling liquid. Acetone dissolves a lot of other organic compounds and can be dissolved in water forever. Because of this and the fact that it has a low boiling point of 56 °C (132.8 °F), it is one of the most important industrial solvents. It has applications in a wide range of products, such as paints, varnishes, resins, coatings, and products that take off nail polish.
Nomenclature
To figure out a ketone's IUPAC name, the parent molecule with the carbonyl group that has the longest carbon chain is chosen. Since it is closer to the end of the chain, the carbonyl carbon in the parent chain is lower on the list. 5-methyl-3-hexanone, for example, has the chemical formula C(H3CH2COCH2CH(CH3)2). The longest chain has six carbon atoms, so we need to start counting from end. This gives the carbonyl carbon a lower number for its carbon number. Carbon 3 hosts the carbonyl group, while carbon 5 is home to the methyl group. In cyclic ketones, the carbonyl carbon is thought to be the atom with the number 1. By adding the word "ketone" to the names of the carbon groups that are covalently bound to carbon, we get the common names for ketones.
Classification
One way to sort ketones is by the types of substituents they can take on. Ketones can be further divided into symmetrical and unsymmetrical derivatives, which make up one large subclass, based on how similar the two organic groups attached to the carbonyl core are. Acetone and benzophenone are both types of ketone with the formula C6H5C(O)C6H5. The ketone acetophenone (C6H5C(O)CH3) is an example of an asymmetric ketone.
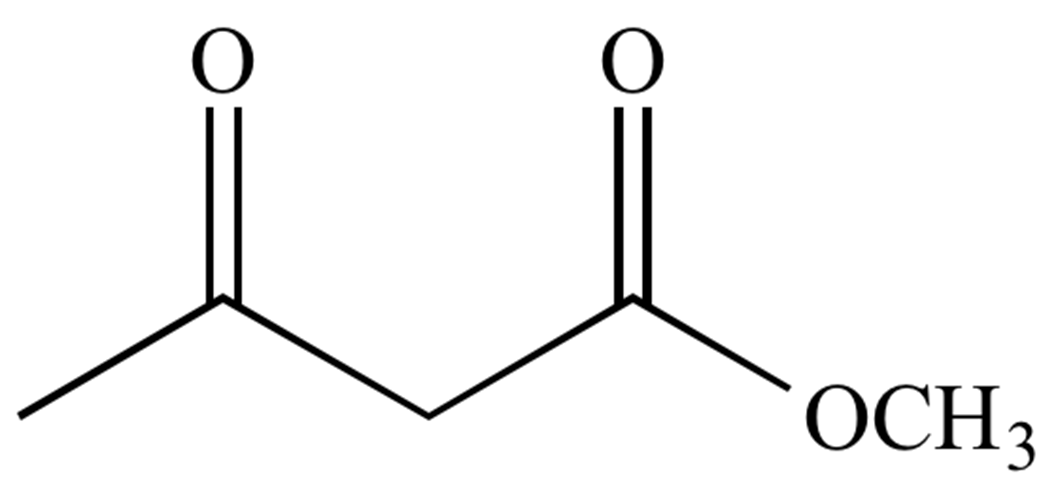
Diketones
There are many different kinds of diketones, and some of them have very strange properties. At first, diacetyl (CH3C(O)C(O)CH3), the simplest of these chemicals, was used to give popcorn butter flavor. Since this compound is most often found as the monoenol CH3C(O)CH=C(OH)CH3, the name acetylacetone (pentane-2,4-dione) is almost certainly wrong. Its enolate ligand is used a lot in coordination chemistry.
Unsaturated Ketones
Ketones that can be broken down further or have more units added to them, like alkene or alkyne, are called unsaturated ketones. Because it is used in the Robinson annulation reaction, methyl vinyl ketone (CH3C(O)CH=CH2) is the most well-known chemical in this group. Because ketones have an unsaturated site, they can be hydrogenated.
Cyclic ketones
A lot of ketones have a structure that looks like a circle. The simplest ones can be written as (CH2)nCO, where n is an integer between 2 (for cyclopropanone) and the tens (for the rest). There are derivatives that are much bigger. The cyclic symmetrical ketone Cyclohexanone is a very important part of the process of making nylon. Isophorone is a ketone that can be made from acetone.
Biochemical Significance
Ketones can be found in many places in nature. During photosynthesis, organic molecules are made with the help of the ketone ribulose-1,5-bisphosphate. Ketoses is another name for the type of carbs called ketones. The most common ketose is fructose, which is usually found as a cyclic hemiketal that hides its ketone functional group. Fatty acids are made with the help of ketones. Through the Krebs cycle, which makes acetoacetate as a middle step, sugar and carbs are broken down into energy.
In the medical field, acetone, acetoacetate, and beta-hydroxybutyrate are all called "ketone bodies." Most vertebrates, including people, can make these chemicals from glucose, fat, and protein. Ketosis happens when ketone bodies build up in the blood after a period of fasting, even if it's just overnight. Ketone bodies are also found in the blood and urine of people who are starving, when hypoglycemia is caused by something other than hyperinsulinism, when a ketogenic diet is used to bring on ketosis, and when diabetes mellitus is the cause of ketoacidosis. Ketosis or even ketoacidosis can happen in people with type 2 diabetes, but it happens more often in people with type 1 diabetes who don't take care of their condition.
Uses
Ketones are mass-produced by industries to be used as solvents, to make polymers, and to make drugs. On a large scale, the three most important ketones are acetone, methylethyl ketone, and cyclohexanone. They are common in organic chemistry and, to a lesser extent, in biology. Ketones and many other types of chemicals are made when hydrocarbons are burned, which is an uncontrolled process of oxidation.
Toxicity
It is hard to say in general terms how dangerous a large group of chemicals are, but simple ketones are usually not very dangerous. Because of this quality, they are often used as solvents. The rule about saturated ketones doesn't apply to methyl vinyl ketone, which has an LD50 of 7 mg/kg and is an exception to the rule (oral).