Methoxy benzene: synthesis and applications
Anisole, commonly known as methoxybenzene, has the molecular formula CH3OC6H5. It does not have an odour, but it has the appearance of anise seed, and some of its derivatives are utilised in fragrances, both natural and artificial. As a scented liquid used in perfume, flavourings, insecticides, and solvents. The molecule is first created through the process of synthesis and then put to use as a precursor in the creation of other synthetic substances. There is such a thing as ether. Since anisole is a commonly used reagent, it may be put to use not only for its practical applications but also for instructional ones.
In its chemical form, methoxybenzene is represented by the formula C6H5OCH3, and its relative density is 0.996. This chemical has both a melting point and a boiling point mentioned; the melting point is -37.5 degrees Celsius, while the boiling point is 155 degrees Celsius.
Its chemical structure can be written as following:
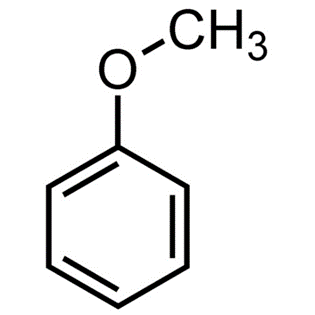
Synthesis
In the Williamson ether synthesis, sodium phenoxide is coupled with a methyl halide to produce anisole. Anisole is the end product of this reaction.
Methylation of sodium phenoxide, either using dimethyl sulphate or methyl chloride, is the first step in the production of anisole.
2 C6H5O−Na+ + (CH3O)2SO2 → 2 C6H5OCH3 + Na2SO4
Two solutions of sodium hydroxide must be used to dissolve two molecules of C6H5O before they can be converted into two molecules of C6H5OCH3 and two molecules of Na2SO4.
Uses
Anisole is used as a precursor in a variety of products, including perfumes, insect pheromones, and pharmaceuticals. An example of this would be dehydrating anisole in order to produce synthetic anethole.
Anisole has a low toxicity; the median lethal dose (LD50) for rats was 3,700 mg/kg, which indicates that it is safe for most applications. The fact that it may quickly catch fire is the most significant threat posed by it.
Trimethoxy Benzene
The methoxybenzene molecule is referred to as 1,3,5-trimethoxybenzene when it has methoxy groups in positions 1, 3, and 5. This biomarker allows for the measurement of flavonoid consumption. It is a possible biomarker as well as a xenobiotic molecule that comes from humans.
The methoxybenzene molecule have the following structural formula:
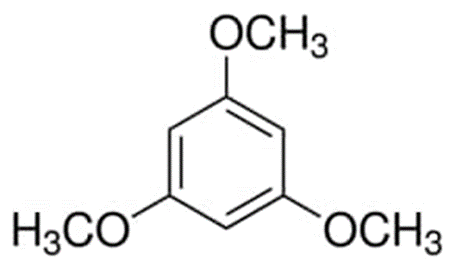
There is a predominant presence of 1,3,5-trimethoxybenzene in the fragrance of Chinese roses. It can be utilised in a range of different cosmetic purposes. In pharmaceutical proton NMR spectroscopy, it was utilised for the purposes of quantitative reference as a secondary reference. Because of this, the p-methoxybenzyl group that is found on the protective side of certain alcohols and acids is cleaved.
Natural 1,3,5-trimethoxybenzene may be found in Zieria chevalieri, Virola surinamensis, and other species for which there are data available. This substance is also known as 1,3,5-Trimethoxybenzene, 621-23-8, phloroglucinol trimethyl ether, sym-Trimethoxybenzene, 1,3,5-trimethoxybenzene, and benzene 1,3,5-trimethoxy. Other names for this molecule include phloroglucinol trimethyl ether.
It begins to melt between 50 and 53 degrees Celsius and begins to boil at around 255 degrees C. (lit.). It does not dissolve in water but does dissolve in methanol, producing a solution that is odourless and transparent after dissolution. This material has a density of 85g/mL with a colour that ranges from white to cream in tone.
Uses
It is common knowledge that taking 1,3,5-trimethoxybenzene can help alleviate the symptoms of pain in the digestive tract and the biliary tract, as well as colic and urinary spasms.** **
- The 1,2,3-trimethoxybenzene molecule has methoxy groups in place of the benzene at positions 1, 2, and 3. The primary role it plays in the world is that of a metabolite in plant life. It is common knowledge that Tetrapanax papyrifer has a naturally occurring component of 1,2,3-trimethoxybenzene.
- In addition to being recognised as an anisole, 1,2,3-trimethoxybenzene is also known as pyrogallol trimethyl ether and methylsyringol. Methoxybenzene and derivatives of methoxybenzene are terms that are used to refer to organic compounds that include anisole. It has a very low basicity (1,2,3-trimethoxybenzene), which means that it is scarcely soluble in water. Additionally, it has a very weak basicity (based on its pKa). Because 1,2,3-trimethoxybenzene is found in tea in its natural state, it has the potential to act as a biomarker for tea consumption.
- Flavoring component that may be found in dried bonito and tea in their natural forms.
Storage
Place in an area that is dry, cool, and has air circulation. Keep it away from any things that might oxidise it and make it less effective.