Methylpyridine: Isomers, synthesis, applications, side effects and storage
Methylpyridine is a pyridine derivative that has the chemical formula C6H7N and a molecular weight of 93.13 g/mol. It is liquid in nature having boiling point of 145°C and auto ignites at 1000°F. A methyl group is added into the original structure of the pyridine molecule to make methylpyridine. According to the chemical abstract services, this substance has been assigned the registration number 1333-41-1.
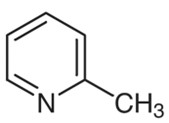
The following is the chemical structure of Methylpyridine:
Isomers of Methylpyridine
There are three different isomers of methylpyridine. Each of these colorless liquids in this category has an odor that is similar to that of pyridine. They are miscible in most of the organic solvents, including water.
The following is a list of the three possible isomers for this chemical:
- 2-Methylpyridine, α-picoline, 2-picoline
- 3-Methylpyridine, β-picoline, 3-picoline
- 4-Methylpyridine, γ-picoline, 4-picoline
The 4-methylpyridine is the most common isomer of this and picoline is another name for this substance.
2- and 4-picolines may be transformed to 2-propenylpyridine by mixing an alkyl methyl group with heated aqueous sodium hydroxide to make 2-propenylpyridine.
We can discuss each of its isomeric form as following:
2-Methylpyridine
It is an organic liquid that has no color or odor. It has a melting temperature of 70 0C, and has a boiling point of 128-1290C (lit.). The flash point of this organic molecule is said to be 97 F0. The organic pyridine molecule may be converted into 2-methylpyridine by performing a 2-C methylation.
Its IUPAC name is 2-methylpyridine and other common names for this compound include 2-Picoline, 109-06-8, o-Picoline, and alpha-Picoline.
It is readily soluble in water, as well as in alcohol and diethyl ether, as well as other solvents.
Synthesis
The 2-picoline is very first pure pyridine chemical that had ever been identified. T. Anderson was the one who extracted it from coal tar in 1846. Reilly Industries utilized this chemical for the first time. At the moment, there are primarily two different methods that it may be produced. In one method, acetaldehyde and ammonia are brought together with the use of an oxide catalyst. The following are examples of 2- and 4-picolines that may be made using this method:
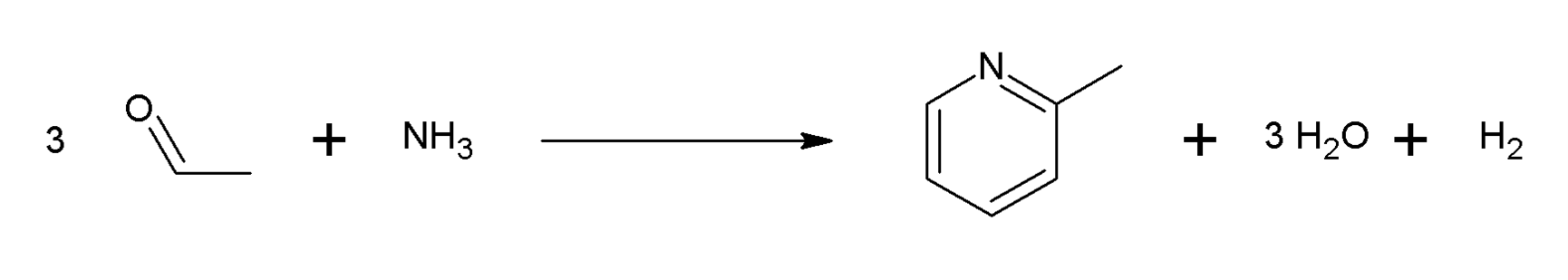
Second, 5-oxohexane may be produced by mixing acetone with acrylonitrile, which then cyclizes to yield 2-picoline as a byproduct.
Uses
In order to investigate the electron and proton transfer mechanisms involving lumiflavin, 2-Picoline (2-Methylpyridine) is utilized in conjunction with Fourier transform ion cyclotron resonance mass spectrometry. In addition to that, it is being utilized in the synthetic method for the production of dearomatized, allylated, and C-H bond activated pyridine derivatives.
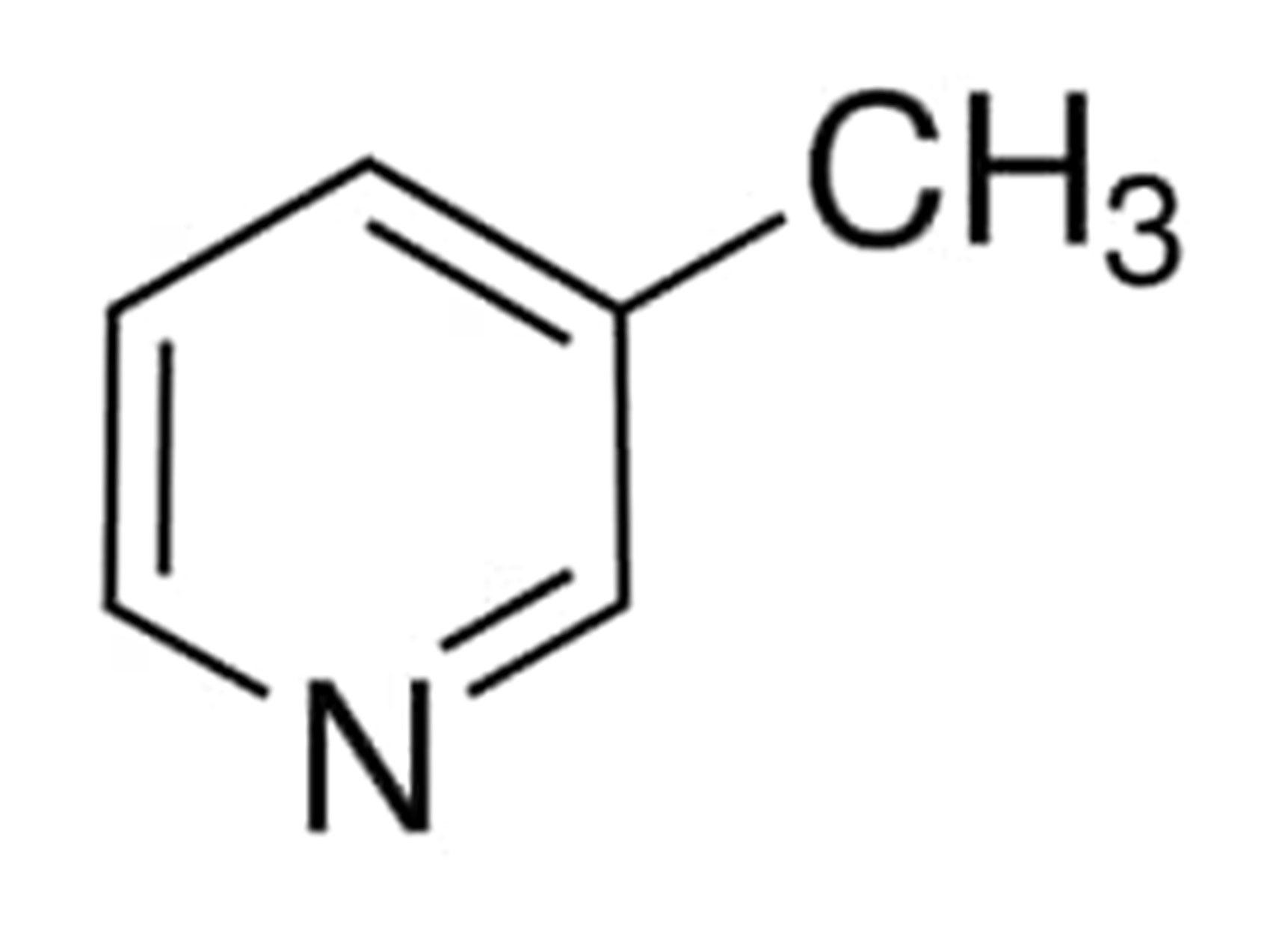
3-Methylpyridine
Adding a methyl group to carbon number 3 leads in the formation of a colorless derivative of pyridine called 3-methylpyridine. This derivative of pyridine has a pleasant aroma that is sweet in nature. It is also known as 3-Picoline, 108-99-6, Beta-Picoline, and 3-Methylpyridine and Pyridine, 3-Methyl.
This material is classified as being among those that are very soluble in water.
The melting point of this compound is −19 °C (lit.) and its boiling point is 144 °C (lit.). The flash point of this compound is known to be 97 °F.
The chemical structure of this compound is as following:
Synthesis
In a process that takes place in the vapor phase over a catalyst that contains nickel, 2-methylglutaronitrile is transformed to 3-methylpiperidine in the presence of hydrogen. Subsequently, 3-methylpyridine is dehydrogenated over palladium-alumina.
Uses
Picolines may be found in a variety of components, including cigarette smoke, bone oil, coal tar, and the emissions that come from coke furnaces. It is used as a solvent and as an intermediate in a number of chemical reactions. Additionally, it is utilized as a flavoring component in a wide range of food and drink goods. As an intermediate in the manufacturing of dyes and resins, it is used to produce insecticides, waterproofing agents, niacinamide, and niacin, among other things.
The manufacturing of agrochemicals and medications is by far the most prevalent use for 3-methylpyridine. Insecticides such as chlorpyrifos, as well as niacin and its amide, food additives, and herbicides such as fluazifop-butyl, can all be produced using chlorpyrifos as an example.
Side Effects
Ingesting it, breathing it, or absorbing it via the skin are all terrible ideas. Damage is caused to the mucous membranes, upper respiratory tract, eyes, and skin by the substance. Inhalation has the potential to be fatal due to spasm, irritation of the larynx and bronchi, chemical pneumonitis, and edoema of the lungs. Some of the indications and symptoms of exposure include a burning sensation in the chest, coughing, wheezing, laryngitis, difficulty breathing, laryngitis, headache, nausea, and vomiting.
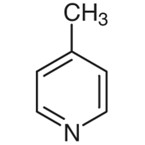
4-Methylpyridine
4-Methylpyridine is a chemical liquid that is odorless and colorless. It has a somewhat high volatility. The synthesis of heterocyclic compounds is possible by employing this liquid as the starting point. The IUPAC name for this compound is 4-methylpyridine. Additionally, it is also known by the following names like 4-Picoline, 108-89-4 Pyridine, and 4-Methylpyridine.
The chemical structure of this compound is as following:
It has boiling point of 292.80C and its melting point is 38.5 F0.
If we talk about its solubility, then it’s miscible in water and it is soluble in alcohol, ether, acetone and carbon tetrachloride.
Uses
It plays an important role in the manufacturing process of isonicotinic acid and the derivatives of that acid. Additionally, it is utilized in the industry of resins as a solvent and in the textile industry as a waterproofing agent.
Storage
It is imperative that glass bottles that contain methylpyridine be stored in a cold environment.