Nitroaniline: Common isomers, structure, synthesis and applications
Aniline
Aniline is the simplest aromatic amine and its chemical formula is C6H5NH2. It is made up of a phenyl group and an amino group that are linked together. Not only does it help make a lot of other chemicals, but it is also a very important part in pharmaceutical industry. It is mostly used as a raw material to make polyurethane, dyes, and other chemicals for industry.
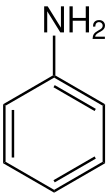
Like many other volatile amines, it smells like rotting fish. It burns with a smokey flame like most aromatic compounds do, and it is easy to set on fire. When people eat it, it makes them very sick. It's got more electrons than, say, benzene. This makes it easier to see how it works in electrophilic aromatic substitution processes. As with other oils, freshly cleaned aniline is almost colorless. However, if it is left out in the air, it gradually turns yellow or red as highly colored, oxidized impurities form.
After aniline has been diazotized and turned into a diazonium salt, it can go through a number of different nucleophilic substitution processes. The root of the word "aniline" is the Portuguese word "anil," which means "indigo shrub." The -ine ending shows that the word comes from "anil." Aniline is both a nucleophile and a base (pKaH = 4.6), though it is less of a nucleophile than aliphatic amines that have the same structure. Some people call aniline dyes "coal tar colors" because the benzene used to make them was first taken from coal tar.
Nitroaniline
Nitroaniline is the name of a chemical compound that is made from aniline (C6H5NH2) and has a nitro group (—NO2) added to it.
Following are three main isomeric forms of nitroaniline:
- 2-Nitroaniline
- 3-Nitroaniline
- 4-Nitroaniline
All of these isomeric forms can be described as following:
2-Nitroaniline
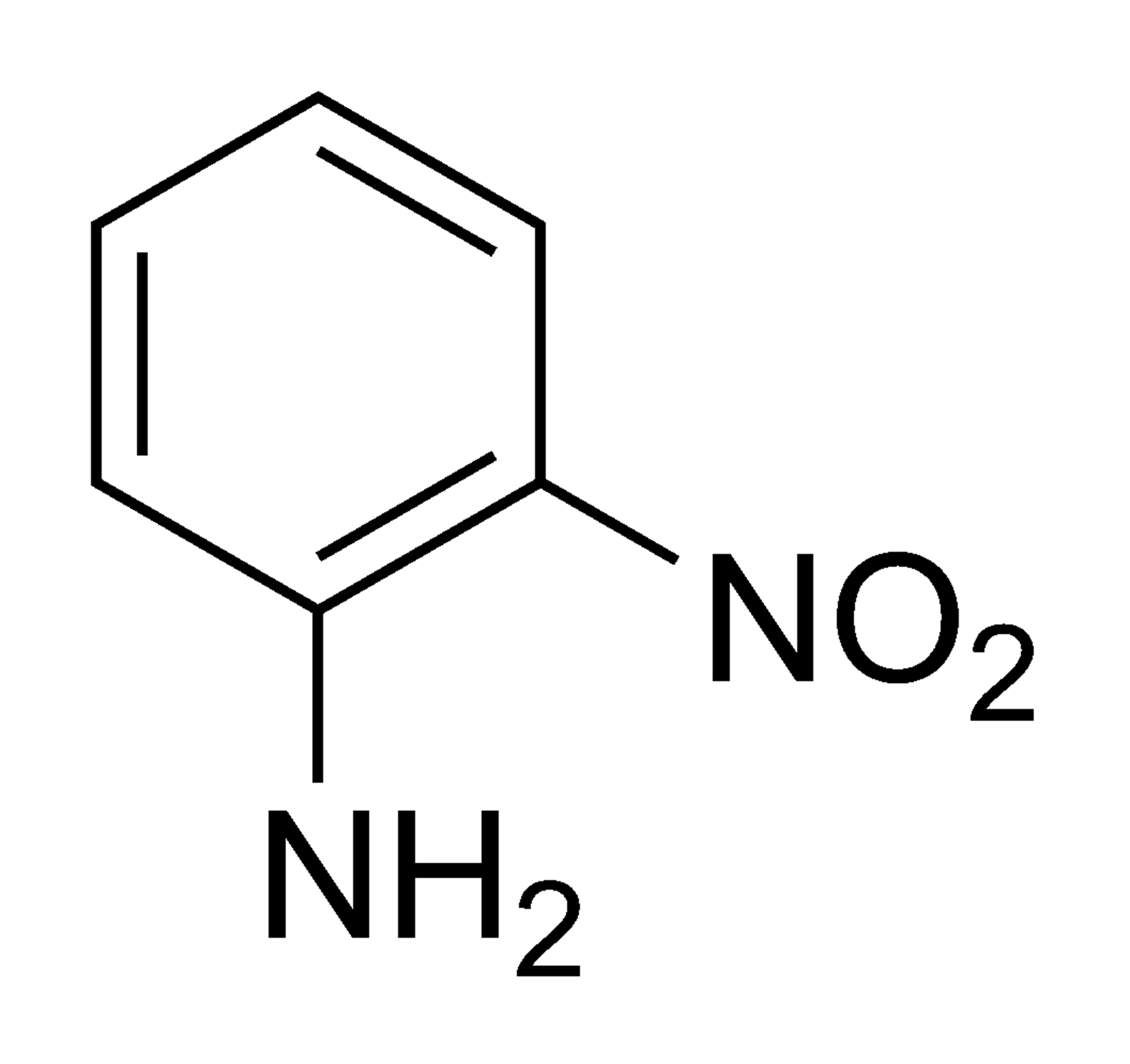
2-nitroaniline's chemical formula is (H2NC6H4NO2). It is related to aniline, but has a nitro group at position 2 instead of the one that is usually there. The main thing it is used for is to make o-phenylenediamine.
It is also called 2-nitroaniline, o-nitroaniline, and ortho-nitroaniline. O-nitroaniline is a solid that is orange and smells musty. The thing sinks and slowly breaks down in water. Under 760 mm Hg of pressure, its melting point is 160.7 °F and its boiling point is 543 °F.
The chemical structure of 2-nitroaniline can be written as:
Synthesis
In order to make 2-nitroaniline commercially, 2-nitrochlorobenzene is made to react with ammonia.
ClC6H4NO2 + 2 NH3 H2NC6H4NO2 + NH4Cl
You can make this chemical in a number of different ways. Direct nitration of aniline, which makes anilinium, is not a good way to do things. When acetanilide is nitrated, not much of the 2-nitro isomer is made. This is because the amide has a strong effect on the sterics of the molecule. Most of the time, sulfonation is used to boost the effectiveness of blocking the 4 location to 56%.
Uses
It is used to make ortho-phenylenediamine, vat dyes, chemicals that keep photos from fogging up, and coccidiostats. It is also used to make medicines, cutting fluids, and chemicals called benzotriazoles.
3-Nitroaniline
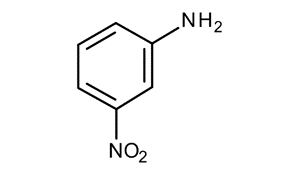
Most dyes start with 3-nitroaniline, which is a stable solid that is sometimes called meta-nitroaniline or m-nitroaniline. 3-Nitroaniline is a type of aniline that has a nitro group attached to carbon 3. It isn't likely to build up in living things, and it doesn't break down quickly in neutral, acidic, or alkaline environments.
The thing smells sweet and burns, and it looks like little yellow needles. It melts at 237 degrees Fahrenheit and boils at 581 and 585 degrees Fahrenheit at 760 mm Hg.
The chemical structure of 3-nitroaniline is written as:
Synthesis
From aniline, m-nitrobenzoic acid is made by first acetylating it, then nitrating it, and then hydrolyzing it to get rid of the acetyl group.
The warm water that has magnesium sulphate in it is treated with 1, 3-dinitrobenzene. The emulsion is broken up by slowly adding an aqueous solution of sodium hydrogen sulphide (6 molar equivalents), then heating it to 90 °C while stirring it constantly. When the 3-nitroaniline is made, it solidifies when it cools. This makes it easy to separate by filtration.
Uses
It is a chemical that is used to make dispersion yellow 5 and acid blue 29, as well as the azo coupling component 17. During the dying process, the chemical is changed into several compounds (dyestuffs and m-nitrophenol).
It can be used to judge the color of pine wood, and it can also be used to make organic compounds.
4-Nitroaniline
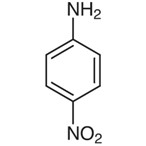
4-Nitroaniline is an organic molecule with the formula C6H6N2O2. It is one of the three isomers of nitroaniline, and it exists as a solid in the yellow spectrum. It is used to stop corrosion, as a color, as a medicine, as an additive to gasoline, to stop gum from sticking, to treat chickens, and for many other things.
The melting point is between 146.0 and 151.0 degrees Celsius, and the boiling point is 332.0 degrees Celsius.
The chemical structure of 4-nitroaniline is:
Synthesis
Industrially, 4-nitroaniline is made by adding an amine to 4-nitrochlorobenzene:
ClC6H4NO2 + 2 NH3 H2NC6H4NO2 + NH4Cl = NH4Cl
Through an electrophilic aromatic substitution, the nitro group is put next to the amino group. When the amino group is charged, it acts as a meta-director. So, there needs to be a way to keep the acetyl group. Small amounts of 2-nitroaniline are also made, and they need to be separated out when the reaction is done.
Uses
It is used to make dye, an antioxidant, a gas gum inhibitor, and a corrosion inhibitor. It is a key ingredient in making a wide range of chemicals, such as those used as building blocks for making dyes, antioxidants, medicines, and insecticides.