Nitrobenzoic acid: Common isomers, application and synthesis
Nitrobenzoic acid is approximately ten times more acidic than the benzoic acid from which they originated. The production of nitrobenzoic acid results from the oxidation of styrene in boiling nitric acid. Nitrobenzoates are the salts and esters of nitrobenzoic acids.
Nitrobenzoic acid may be found in three distinct isomers, which are as follows:
- 2-nitrobenzoic acid
- 3-nitrobenzoic acid
- 4-nitrobenzoic acid
All of these isomeric forms can be discussed in detail as following:
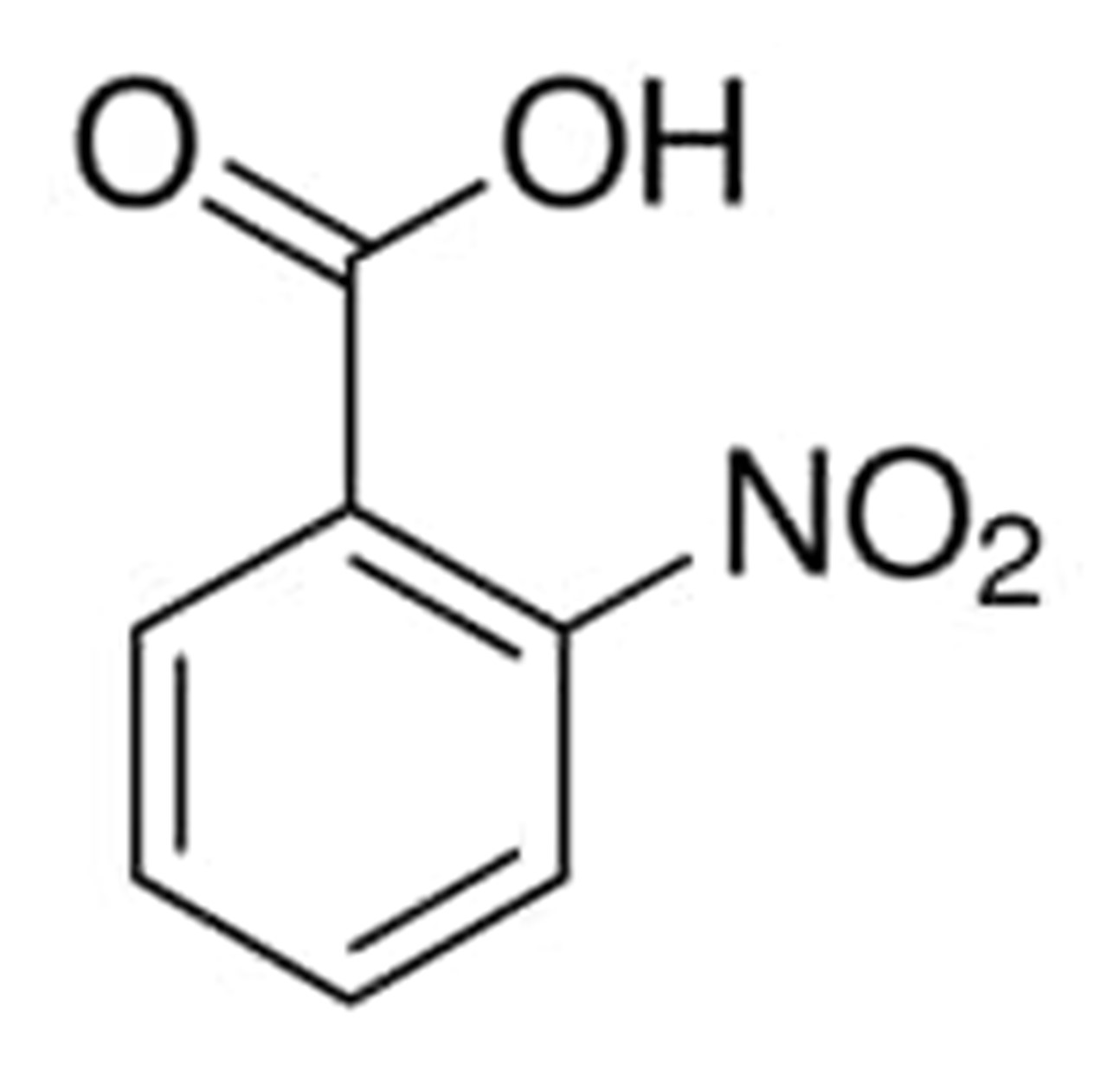
2-Nitrobenzoic acid
2-Nitrobenzoic acid is an isomer of nitrobenzoic acid that has a nitro substituent positioned in the number 2 position. It is one of the conjugate acids. It is a molecule that is organic in nature and has the chemical formula C6H4(NO2)CO2H. The very first step in order to make it is for nitric acid to oxidize some 2-nitrotoluene.
It is present in the form of crystals that are yellow-white in color and have a very sweet flavor. It is insoluble in water and melts between 297 and 298 degrees Fahrenheit. In addition, it is known as 2-Nitrobenzoic acid, trimethylsilyl ester; 2-Nitrobenzoic acid, and Trimethylsilyl 2-nitrobenzoate. All of these names refer to the same substance.
The chemical structure of 2-nitrobenzoic acid can be written as:
Uses
- It is used in the process of organic synthesis, both as a reagent and for the purpose of amine group preservation.
- It is utilized both as a growth supplement and as a synthesis reagent by the KU-7 strain of Pseudomonas fluoresces.
- Additional uses include the maintenance of amine groups and the utilization of these groups in chemical synthesis.
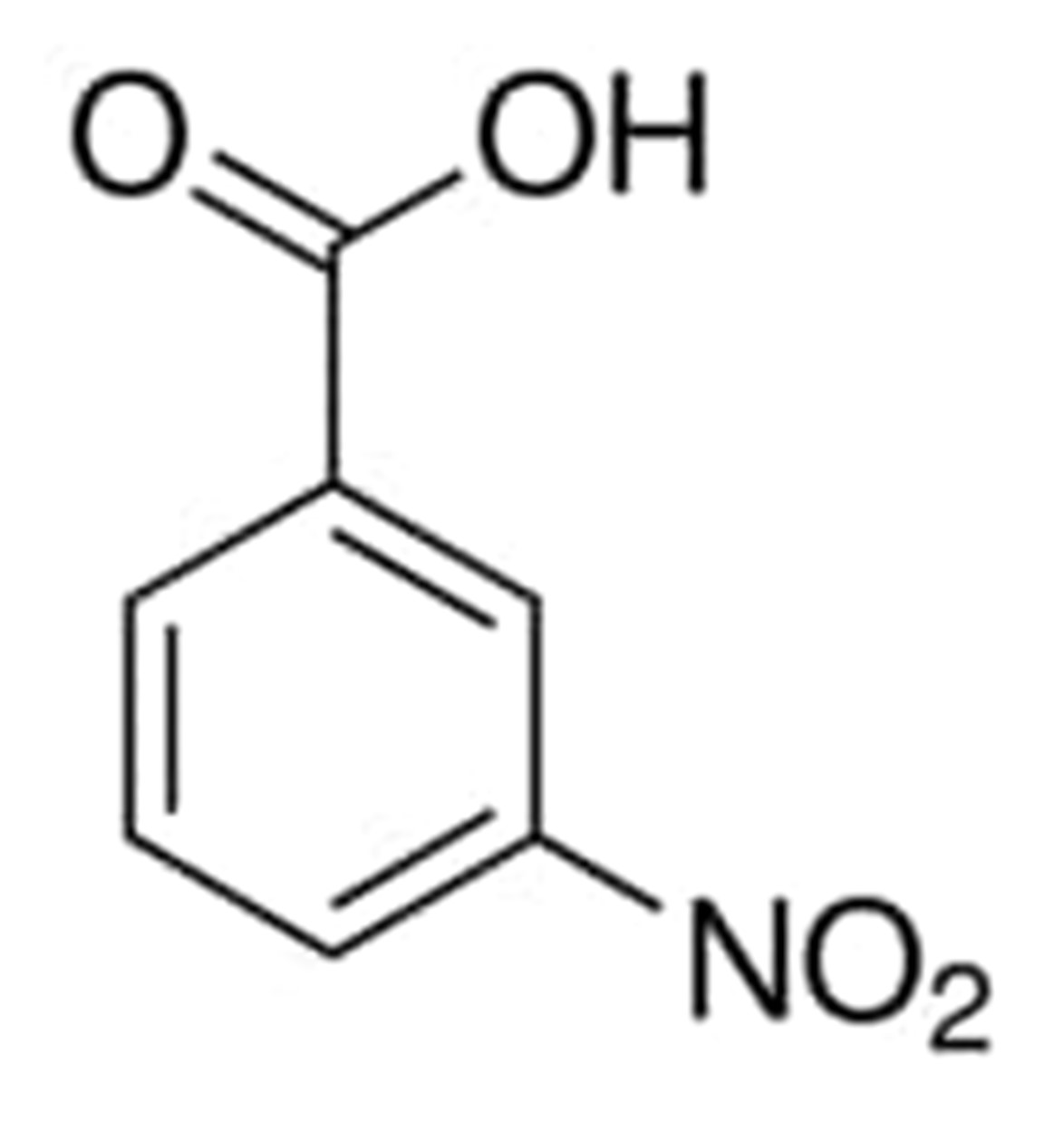
3-Nitrobenzoic acid
Crystals of 3-nitrobenzoic acid have a flavor that is described as being bitter and have a color that ranges from off-white to yellowish-white. The melting point of 3-nitrobenzoic acid is increased by hot water. You might know it better by its alternative moniker, 3-nitrobenzoic acid.
The temperature at which sodium meta-nitrobenzoate, also known as 3-nitrobenzoic acid, will begin to melt is somewhere in the range of 284 to 288 degrees Fahrenheit.
The chemical structure of 3-nitrobenzoic acid can be written as:
Uses
It is utilized in the manufacture of intermediates, the synthesis of alkaloids, and the synthesis of thorium, among other processes. Additionally, it is utilized in the preparation of 5-amino-2-hydroxy benzoic acid.
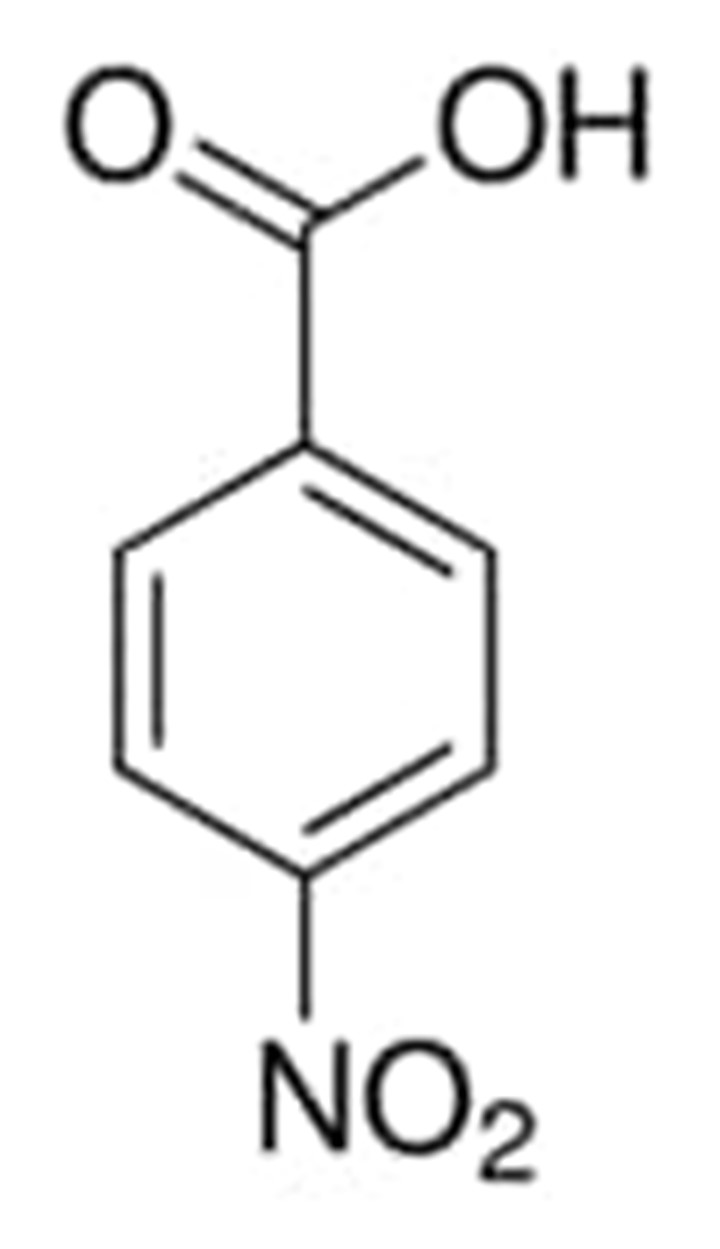
4-nitrobenzoic acid
P-nitrobenzoic acid is the parent compound of its derivative, the carboxylic acid known as 4-nitrobenzoic acid. To be present in the biosynthetic process, it is necessary for the production of the antibiotic aureothin. Molecule of 4-nitrobenzoic acid adsorb on the surfaces of fine silver powder as carboxylate, as determined by infrared Fourier transform spectroscopic investigation.
The chemical structure of 4-nitrobenzoic acid can be written as:
Uses
- It is possible to make use of 4-nitrobenzoic acid in the process of producing compounds (I) and (III) trans-M2(T(i)PB)2L2, in which T(i)PB stands for 2,4,6-triisopropylbenzoate.
- In addition to being a reagent for alkaloids and thorium, it is utilized in the production of medicines and colors.
- One further use is in organic synthesis.
- It is also used in the production of nitrobenzoic acid
Nitrobenzoic acid synthesis
- It is necessary to nitrate benzoic acid at a low temperature in order to produce 3-nitrobenzoic acid. About twenty percent of each of the 2-nitro and 4-nitro isomers are produced as a byproduct of the reaction. The purification of 3-nitrobenzoic acid can be accomplished effectively by the process of recrystallization of its sodium salt. In order to get a higher overall yield, the oxidation of benzaldehyde into 3-nitrobenzaldehyde must take place under strict laboratory conditions.
- The essentially isomer-free nitrobenzoic acids are vital components, since they play an important role as intermediates in the production of pharmaceuticals, dyestuffs, and monomeric polymerizable compounds. This technique of manufacturing 2- or 4-nitrobenzoic acid is inefficient due to the formation of the more practical 3-nitrobenzoic acid during the nitration of benzoic acid.
- This invention aims to manufacture 2- or 4-nitrobenzoic acid without the presence of its isomer, in yields that are acceptable, and without the need for tedious isolation techniques. This objective can be accomplished via the use of this invention. Additionally, the idea seeks to simplify the process of converting 2- or 4-nitroacetophenone into 2- or 4-nitrobenzoic acid.
- When the respective ketones are treated with nitric acid, 2- or 4-nitrobenzoic acid is produced. This results in the accomplishment of the goals outlined above as well as the goals that will be covered in the next section.
- The following is a rundown of the fundamental processes involved in the transformation of 2- or 4-nitroacetophenone into 2- or 4-nitrobenzoic acid: In order to convert 2- or 4-nitroacetophenone fully into 2- or 4-nitrobenzoic acid, nitric acid is added to the ketone first, and then the mixture is heated until the ketone is totally transformed. Because the emission of nitrogen dioxide is a side consequence of the oxidation, it may be used to establish when the reaction originally began and when it finally finished. This is because nitrogen dioxide is a trace gas that can be detected quite a distance away. It takes eight moles of nitric acid to convert one mole of nitroacetophenone. However, while it is most effective to make use of the nitric acid in the stoichiometric quantity, it is feasible to make use of either more or less of the nitric acid.
- The use of a nonreactive diluent, such as water, can facilitate the running of a reaction more smoothly. Nitric acid diluted in water is the most powerful type of oxidant. The ideal temperature for the reaction ranges from around 60 degrees Celsius up to the point when the reaction mixture begins to reflux; however, this varies depending on the concentration of the nitric acid that is being employed.
- The beginning stages of the oxidation process are typically rather slow. We find that working in the presence of a reducible metal compound in a little quantity, say between 0.5 and 2 percent, depending on the weight of the nitroacetophenone, is the most effective way to shorten the length of time required for this induction phase. A variety of substances, including ammonium metavanadate, potassium permanganate, ferric oxide, mercuric oxide, sodium dichromate, and others, can be utilized to reduce the amount of time required for the induction process.
- Using methods that are common in the industry, the two nitrobenzoic acids may be easily extracted from the final reaction mixture and used separately. In most cases, the crude acid will crystallize once the reaction mixture has been cooled, at which point it may be filtered out of the mixture and used. After that, the crude product might be refined by recrystallization from various solvents such benzene, ethanol, mixtures of benzene and ethanol, hexane, and so on.