Nitropyridine: Synthesis, reactions, applications, side effects and storage
The nitrated forms of pyridines are referred to as Nitropyridines. Its most common isomeric form is 2-Nitropyridine.
2-Nitropyridine is a pyridine derivative that is also referred to as -nitropyridine and pyridine, 2-nitro-. 2-Nitropyridine is a derivative compound of pyridine that possesses a nitro group. Its chemical formula is written as C5H4N2O2. 2-Nitropyridine has a molecular weight of 124.10 grammes per mol. of powder.
Its IUPAC name is 2-Nitropyridine and is also known by the names such as Pyridine, 2-nitro-, Pyridine, nitro-, 2-Nitropyridine 97 percent, Nitro-pyridine and 2-nitro-pyridine.
The following diagram depicts the general formula of Nitropyridine:
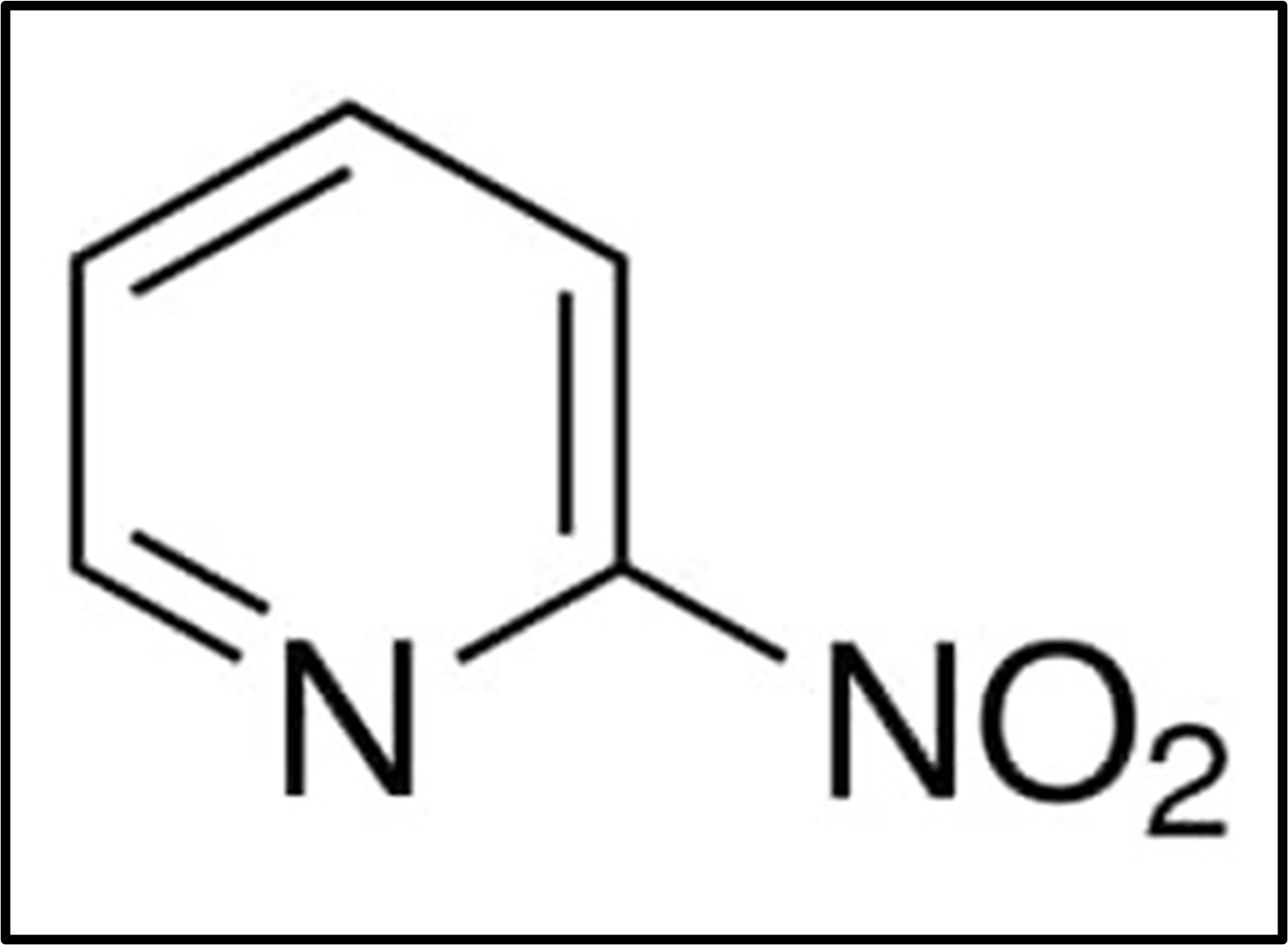
The melting point of 2-Nitropyridine is between 35 and 40 degrees Celsius and it has a flash point of 107.2 degrees Celsius. According to Chemical Abstracts Service, the registration number for this compound in the CAS registry is 56778-64-4.
Researchers in the chemical and biological fields have shown a great deal of interest in these compounds due to the strong polarity, better oxidizing characteristics and capacity to simultaneously accept and give charges that they possess.
Because the electron-rich N-oxide functional group is capable of participating in coordination interactions such as hydrogen bonding and metal chelation, as well as performing the functions of bridge chromophores and – push–pull molecular rectifiers, the versatility of N-oxide hetero arenes can be attributed to this ability.
Changing the pyridine-N-oxide functional group to a strong electron-withdrawing entity, such as the NO2 functional group, will undoubtedly change the charge density distribution and structural characteristics of the heterocyclic system, which will ultimately result in an increase in the system's reactive potential.
Synthesis
To nitrate the compounds that belong to this class is the most prevalent approach that is taken while attempting to synthesize nitropyridines. On the other hand, this reaction can only take place in pyridines that include electron-donor substituents.
If the nitropyridines being manufactured have a significant amount of electron-acceptor substituents, then it will not be possible to make the nitropyridines using this approach. Using amines that are a good match for the structure, it is feasible to synthetically produce molecules with this structure. When aminopyridines are directly oxidized with electron-acceptor groups, on the other hand, the formation of nitro derivatives does not take place. The treatment of pyridines with N2O5 in water containing liquid SO2 and water at a temperature of -11 degrees Celsius is possible, and the reaction mixture may then be emptied into water.
In addition, in order to synthesise 2-nitropyrimidines, the sulfilimino and nitro groups need to be oxidized in the correct order. A procedure quite similar to this one can be utilized in order to convert aminoazines into nitro derivatives.
Pyridine Reactions to make various Nitropyridines
In the presence of an organic solvent, the formation of N-nitropyridinium ion takes place when pyridine and substituted pyridines react with N2O5. This substance, when exposed to water, reacts with SO2/HSO3- to produce 3-nitropyridines (77 percent yield). This method produces low yields of 3-substituted compounds, however it produces large yields of 4-substituted compounds when it comes to substituted pyridines. This is not an example of an electrophilic aromatic substitution; rather, a sigmatropic shift in the chemical reactions causes the nitro group to transfer from the 1-position to the 3-position.
Step one in the production of 5-nitropyridine-2-sulfonic acid is the production of 3-nitropyridine. As a consequence of this, a wide range of 2-substituted 5-nitropyridines have been produced. Using the vicarious nucleophilic substitution (VNS) and the oxidative substitution technique at the position para to the nitro group, respectively, it is possible to substitute ammonia and amines in place of the nitro group in 3-nitropyridines. This can be done by employing the techniques described in the previous sentence.
Uses
The asymmetric allylation of aldehydes is one of the many chemical and biological processes that can benefit from the usage of nitropyridine-N-oxide and its derivatives.
In agricultural settings, it is standard practice to use nitrogen pyridine-N-oxides for the purpose of controlling plant development.
It is possible to obtain antifungal treatment by using it.
These substances are regarded to be genotoxic due to the fact that they are capable of causing damage to DNA by interfering with enzymes that are involved in the replication of DNA. Additionally, there is a possibility that cell mutations might occur, which could ultimately result in cancer.
Side effects
If this chemical is eaten, it is potentially dangerous, and if it is taken orally, it may create significant issues.
It is harmful if it comes into contact with the skin. It has the potential to cause substantial discomfort and even corrosion when it comes into contact with the skin.
If it gets into your eyes, it is likely to be highly irritating, and it also has the potential to do significant damage.
Even a single exposure, if the material is breathed in, can be enough to cause damage to the lungs and other parts of the respiratory system if it is inhaled.
Storage
This white powder needs to be kept at a temperature below 30 degrees Celsius.