Nitros: Synthesis, occurrence, reactions and applications
Nitro compounds are those that have one or more nitro functional groups (NO2) in them. The nitro group is one of the most common and most often used examples of a functional group that gives a molecule explosive properties. The nitro group is also a good group for taking electrons away. This property suggests that C—H bonds next to the nitro group, which are also called "neighboring bonds," may become acidic. In addition to these effects, when nitro groups are present in aromatic compounds, the rate of electrophilic aromatic substitution slows down while the rate of nucleophilic aromatic substitution speeds up. In nature, it is rare for something to have a nitro group. In the nitration process that makes them, nitric acid is almost always the first thing that is added.

Synthesis
Most aromatic nitro compounds are made in a lab using a process called nitration. By mixing nitric acid and sulfuric acid, the electrophilic nitronium ion (NO+2) is made, which is then used in the nitration process.
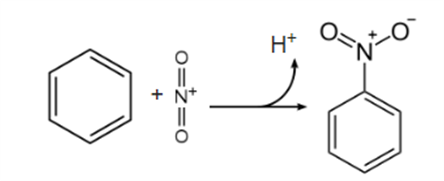
Nitrobenzene is the most common nitration product in the world because it has few competitors. Nitration is a chemical process that can be used to make several different kinds of explosives. Trinitrophenol (picric acid), trinitrotoluene (TNT), and trinitroresorcinol are some examples of these (styphnic acid). The aryl-NO2 group can also be made from halogenated phenols through a process called Zincke nitration. However, this step requires more technical knowledge and specialized equipment.
Preparation of Aliphatic Nitrogen Compounds
There are many ways to make aliphatic nitro compounds, but some of the most common ones are:
- Nitration of alkanes by electrons that don't have a pair. When nitric acid is added to propane in the gas phase (for example, at 350–450 °C and 8–12 atm), a wide range of products are made. These include nitromethane, nitroethane, 1-nitropropane, and 2-nitropropane, which are all fragments of the parent alkane. With nitric acid, propane is turned into a gas to make these chemicals.
- When halocarbons or organosulfates mix with silver or alkali nitrite salts, the process is called a "nucleophilic substitution reaction."
- In the lab, you can mix sodium chloroacetate with sodium nitrite to make nitromethane.
- Oxidation of oximes or primary amines.
- Through the decarboxylation of nitrile and ethyl nitrate, -nitro carboxylic acids are made.
Occurrence
Chloramphenicol is one of the few nitro compounds that can be found in nature. Some of the nitro groups found in nature came from the oxidation of amino groups. A pheromone called 2-nitrophenol helps a lot of ticks get together.
Rarely do you find nitro compounds in nature. Organic molecule 3-nitropropionic acid is found in plants and fungi (Indigofera). Termites make nitropentadecene, which is used as a chemical in defence. In Aniba canelilla, nitrophenylethane was found not too long ago. It turns out that nitrophenylethane can also be found in plants from the Annonaceae, Lauraceae, and Papaveraceae families.
Reduction
Nitro compounds are involved in a number of organic reactions, the most important of which is their conversion to amines. When hydrogen gas is added to nitrogen oxides, it turns them into ammonia and water.
Acid-base Reactions
The carbon in nitroalkanes is only a little bit acidic. The pKa of dimethyl sulfoxide (DMSO) is 16.9, so the pKas of nitromethane and 2-nitropropane in its solution are both 17.2. Based on what we've seen, a pKa in water of around 11 makes sense. Because of this, these carbon acids can be deprotonated in water. The intermediates of both the nitroaldol reaction and the Nef reaction are nitronates. People call them the conjugate base.
Biochemical Reactions
The nitroaldol reaction is when nitromethane is added to an aldehyde through a 1, 2-addition that is sped up by a base. It can also take part in the 1, 4-addition reaction between alpha- and beta-unsaturated carbonyl compounds because it is a Michael donor. Nitroalkenes are the molecules that take part in the Michael reaction between enolate molecules and nitroalkenes.
Flavin is needed by several enzymes to turn aliphatic nitro compounds into safer aldehydes and ketones. nitroalkane oxidase and 3-nitropropionate oxidase are the only enzymes that can oxidize aliphatic nitro compounds. Other enzymes, such as glucose oxidase, can oxidize a wide range of physiological substrates.
Uses
Redox reactions cause organonitro compounds to blow up when they break down. In these reactions, the oxidant (the nitro group) and the fuel are both part of the same molecule (the hydrocarbon substituent). During an explosion, heat is made when molecules that are very stable are put together. Some of these are nitrogen gas (N2), carbon dioxide (CO2), and water (H2O). This redox reaction is more likely to explode because even at low temperatures, its stable products are gases. The nitro group is found in a lot of contact explosives.