Phenylenediamines: Common isomers, synthesis, applications and safety hazard
Phenylenediamines are made from aniline, but they are different from aniline. Phenylenediamine is found in all black dyes, including those used for permanent hair colors, leather, printer's ink, fax machine ink, and lithography. They can cause contact dermatitis. Teenagers are getting more and more temporary black tattoos, which has led to a higher risk of sensitization among this age group.
In the field of coordination chemistry, phenylenediamine is one of the most important ligand precursors. Schiff base ligands that are made from salicylaldehyde work very well for chelation. By oxidizing its metal-phenylenediamine complexes, you can make diimine derivatives. These compounds are often very colorful and can be in a wide range of stable oxidation states.
There are three main isomeric forms of Phenylenediamines:
- o- Phenylenediamines
- p- Phenylenediamines
- m- Phenylenediamines
All of these isomeric forms can be discussed as following:
*o-*Phenylenediamines
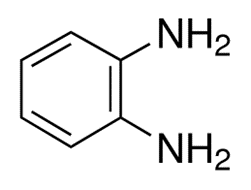
The chemical formula for the chemical compound o-Phenylenediamine, is C6H4(NH2)2. This aromatic diamine is a building block for many heterocyclic compounds. Both m- and p-phenylenediamine isomers are almost the same as this one.

If o-phenylenediamine is pure, it forms colorless monoclinic crystals. If it isn't pure, it looks like a solid that is either brownish-yellow or sandy-brown, depending on the quality. It is used to make dyes, take pictures, and make organic compounds. It melts at 216 to 219 °F and its boiling point ranges between 493 to 496 °F at 760 mm Hg.
The chemical structure of o-phenylenediamine could be written as:
Uses
It is used as an intermediate for the synthesis of various medicinal compounds such as:
- Tiabendazole
- Pyrazinamide
- Morinamide
- Clemizole
- Chlormidazole
It changes into benzimidazole when it comes into contact with carboxylic acids and their derivatives. Quinoxalinedione and mercaptoimidazoles are made by reacting dimethyl oxalate or xanthate esters with dimethyl oxalate or xanthate esters. After a reaction with nitrous acid turns it into benzotriazole, it can be used to stop corrosion. By reacting with different diketones, substituted o-phenylenediamine is an important part of making medicines.
*p-*Phenylenediamine
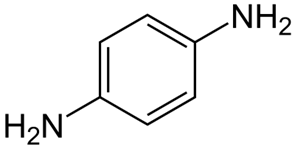
*p-*Phenylenediamine is a chemical compound with the formula C6H4(NH2)2. This chemical is similar to aniline. At room temperature, it is colorless, but when it is exposed to air, it can change color. It is mostly used to make technical polymers and composites, such as Kevlar. It is also sometimes used to color hair instead of henna.
At 760 mm Hg, it boils at 513 degrees Fahrenheit and melts at 284 degrees Fahrenheit.
Following diagram depicts the chemical structure of *p-*Phenylenediamine:
It is dangerous whether it is eaten, breathed in, or absorbed through the skin. This chemical can be used to make aramid fiber, antioxidants, laboratory reagents, photographic development, and hair/fur color, among many other things.
Synthesis
*p-*Phenylenediamine can be made in three ways. Most of the time, 4-nitrochlorobenzene is treated with ammonia and then hydrogenated to make 4-nitroaniline as shown in the chemical reaction given below:
ClC6H4NO2 + 2 NH3 H2NC6H4NO2 + NH4Cl
When we mix H2NC6H4NO2 and 3 H2, we get H2NC6H4NH3 and 2 H2O.
In the DuPont method, aniline is turned into diphenyltriazine first, and then diphenyltriazine is turned into 4-aminoazobenzene with the help of acid catalysts. By adding hydrogen to the latter, PPD can be made.
Hazards
Para-phenylenediamine can cause urticaria, or inflammation in one spot. Angioneurotic edoema is a sign of being poisoned by *p-*phenylenediamine, and it can show up anywhere from 2 to 24 hours after exposure.
In the early 1930s, a product called Lash Lure came out on the market in the United States. Lash Lure was an eyelash dye that had the chemical p-phenylenediamine in it. Because this product hadn't been tested for eye irritation before it hit the market, it caused corneal ulcers in a number of people, which hurt their eyesight for good.
Acute (short-term) exposure to high amounts of p-phenylenediamine in humans has been linked to severe dermatitis, eye irritation and tears, asthma, gastritis, kidney failure, vertigo, tremors, convulsions, and coma. Eczematoid contact dermatitis can be caused by long-term contact with people. When rats and mice were fed p-phenylenediamine for a long time, they got smaller, but they didn't show any other signs of being sick. We don't know what effects p-phenylenediamine has on how humans reproduce, grow, or get cancer. The Environmental Protection Agency has not said how likely p-phenylenediamine is to cause cancer.
m-Phenylenediamine
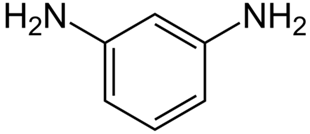
The chemical formula for the molecule of m-phenylenediamine is C6H4(NH2)2. This chemical is also called 1, 3-diaminobenzene. This isomer is the same for both o- and p-phenylenediamine. This aromatic diamine is a solid that looks like needles. When it is exposed to air, it turns red or purple. Samples are often sent as white flakes that may change color after being stored.
Crystals of m-phenylenediamine are either colorless or white, but when they come into contact with air, they turn a bright red or purple. Between 64 and 66 degrees Celsius is its melting point. Density of 1.14 g/cm3. There is a 280-degree Fahrenheit flash point. Eyes and skin can get irritated. It is dangerous whether it is eaten, breathed in, or absorbed through the skin. Used to make aramid fibers, as a polymer additive, to make dyes, as a chemical reagent, and to take pictures.
Its chemical structures can be written as follows:
Uses
m-Phenylenediamine can be used to make the following compounds:
- Poly (m-phenylenediamine-co-2-hydroxy-5-sulfonic aniline) microparticle semiladder structures have been proven to have semiconducting electrical characteristics on their own.
- Using a chitosan-poly (m-phenylenediamine) (CS-PPD) Fe3O4 nanocomposite as a sorbent for magnetic solid-phase extraction (MSPE) of polychlorinated biphenyls.
- With chemical oxidative polymerization, terpolymers are made one after the other.
- TFC membranes made of thin films made of polyamide
- Membranes for reverse osmosis (RO) systems that use TFC