Pyridine as anti-antioxidant agent
Because of their many biological actions, heterocyclic compounds are critical in the discovery and development of novel drugs. Without the usage of pyridine derivatives, medical chemistry would not be the same. Pyridine derivatives are found in many vitamins, enzymes, proteins, and nucleic acid bases, suggesting their extensive role in biological systems. Most 2-amiopyridine derivatives have antibacterial, antifungal, cardiotonic, antimicrobial, pain relievers, anti-inflammatories, antioxidant, and cancer-fighting activities.
4-(4-chlorophenyl)-2-aryl substituted metheniminothiazoles acts on free radicals
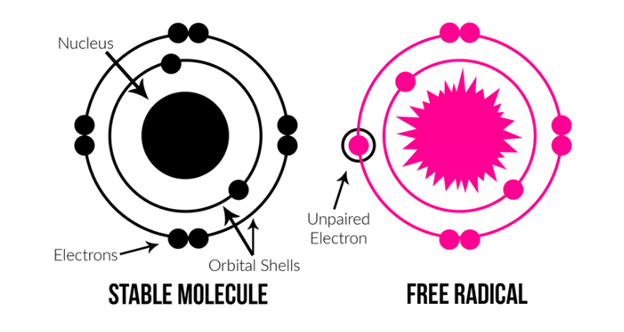
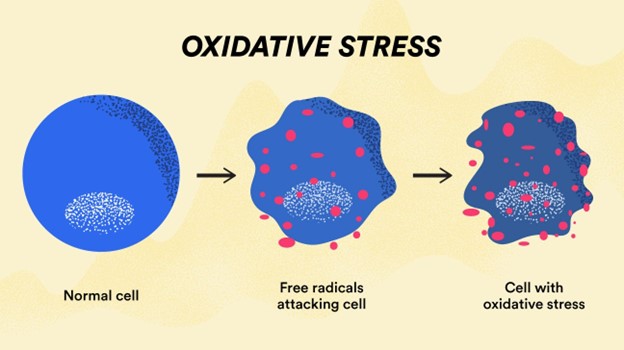
Oxygen-centered free radicals such as superoxide, nitric oxide, and hydroxyl are examples of reactive oxygen species. Endogenous lipid, protein, and DNA damage has been associated to oncology, drug-related toxicity, and inflammation, among other illnesses. Furthermore, radical reactions have an important role in the development of cancer, ageing, diabetes, and other diseases throughout time. Diseases associated with oxidative stress have been demonstrated to react well to antioxidant therapy, and this treatment has been proved to be both safe and effective. Consuming antioxidant-rich meals protects against neurological and cardiovascular disease in addition to decreasing cancer risk. Thiazole compounds have recently been revealed to exhibit antioxidant action (AOA). Kavitha et al. discovered that several novel 4-(4-chlorophenyl)-2-aryl substituted metheniminothiazoles had significant antioxidant capabilities.
Antioxidant organoselenium compounds reduces oxidative stress
Antioxidant organoselenium compounds include: CuI/SeO2 catalysed the selective synthesis of 3-(organylselanyl)-1 H-indoles and 3-(organylselanyl) imidazole [1,2-a]pyridines under ultrasonic irradiation. A battery of in vitro assays were used to assess antioxidant activity, and this method employs a wide range of 1 H-indoles or imidazo[1,2-a]pyridines and diorganyl diselenides to selectively create the relevant products in good to outstanding yields. Bioassays revealed that the two groups of newly synthesized organoselenium compounds exhibit antioxidant action, particularly the 3-(organylselanyl)-1 H-indoles. This pharmacological activity has various potential biological applications since it reduces oxidative stress.
Anti-oxidant activity of 5,7-dimethyl-2-oxo-thiazolo[4,5-b]pyridine-3-yl)-acetic acid
A work describes the synthesis and antioxidant activity of many novel (5,7-dimethyl-2-oxo-thiazolo[4,5-b]pyridine-3-yl)-acetic acid hydrazide derivatives. The base heterocyclic was altered by the steps of acylation, [2+3] cyclocondensation, Knoevenagel condensation, and alkylation to produce compounds with an acceptable pharmacological profile. The capacity of each chemical synthesized to quench 2,2 diphenyl-1-picrylhydrazyl (DPPH) radicals in vitro was examined for antioxidant capabilities.
Examples of antioxidant pyridines
Anti-oxidant activity of pyridine-based chalcones
In a newly synthesized series of pyridine-based chalcones, antioxidant capacity was assessed utilizing beta carotene bleaching (BCB), DPPH free radical scavenging, ferrous ion chelating (FIC), and Trolox equivalent antioxidant capacity (TEAC). Each chemical was produced at room temperature using an aldol condensation process in either methanol or ethanol, and then characterized using 1HNMR, IR, and MS spectroscopic methods. Each substance's melting points were also established. According to the findings, pyridine-based chalcones (3a-3j) might be presented as prospective antioxidant lead compounds.
N-((3,5-dimethyl1H-pyrazol-1-yl)methyl)pyridin-4-amine derivatives and free radicals
Free radicals generate oxidative stress, which damages cell membranes, membrane lipids, and nucleic acids. This transition occurs in the ageing pathogen, as well as cancer, atherosclerosis, and Alzheimer's disease. As a result, there has been a lot of interest in discovering strategies to get rid of free radicals and other related species in recent years. The most interesting chemicals are heterocyclic molecules such as 1,3-thiazole, a nitrogen- and sulfur-containing heteroatom-containing five-membered ring. Numerous studies have investigated at the antioxidant activity of heterocyclic compounds, and the N-((3,5-dimethyl1H-pyrazol-1-yl)methyl)pyridin-4-amine derivatives are the ones that approach closest to this scaffold.3.
Fe(II), Ni(II), and Pd(II) complexes showing anti-oxidant property
A novel Schiff base ligand containing a pyridine moiety, for example, was employed to create a variety of Fe(II), Ni(II), and Pd(II) complexes. The compounds were described using FT-IR, 1H NMR, 13C NMR, UV-Vis, powder XRD, thermogravimetric analysis, mass spectra, magnetic susceptibility, and elemental analysis. In octahedral coordination geometry, oxygen atoms from the carbonyl group, nitrogen atoms from the azomethine moiety, and oxygen atoms from the phenolic group all coordinate the Fe(II) and Ni(II) metal ions. The Pd(II) complex has a square planar shape. All generated compounds were tested for biochemical properties such as enzyme inhibition and antioxidant activity. When examined in vitro using the DPPH and FRAP antioxidant procedures, the Schiff base ligand and its Fe(II)/Pd(II) complexes demonstrated antioxidant characteristics similar to the standards (BHA, BHT, ascorbic acid, and -tocopherol).
Quaternary ammonium compounds of pyridine acts as anti-oxidant
A novel family of quaternary ammonium compounds was produced by chemically modifying chitosan. Among these compounds were pyridine and amino-pyridine. Chitosan and synthetic chitosan versions were tested for antioxidant capabilities. Synthetic chitosan derivatives outperformed chitosan in terms of water solubility and antioxidant activity in all experiments. The inhibition of hydroxyl- and DPPH-radical production is demonstrated here, suggesting that the pyridine functional group is involved in the biological activity. Furthermore, the antioxidant activity of the generated chitosan versions with an amino group at the polymer's perimeter was greater. Antioxidant activity of chitosan derivatives can be enhanced by a synergistic interaction between the amino group and pyridine. Furthermore, it is reasonable to believe that the placement of the amino group on pyridine may impact the antioxidant effect of these chitosan derivatives.
Anti-oxidant activity of ruthenium (II) complexes
The antioxidant activity of the ligands and their ruthenium(II) complexes was determined using DPPH radical, nitric oxide radical, hydroxyl radical, and hydrogen peroxide scavenging techniques, and the findings suggest that the ruthenium(II) complexes are more powerful than the ligands alone. The capacity of the compounds to scavenge 2,2'-diphenyl-1-picrylhydrazyl (DPPH) radicals was used to assess their antioxidant efficacy. The lower IC50 value (2.27 g/ml) established using probit analysis in SPSS 16 indicates the ligand's good antioxidant capacity.
1,1-diphenyl-2-picrylhydrazyl (DPPH) as anti-oxidant
The capacity of the compounds to scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH) was evaluated to determine their antioxidant activity. Strong antioxidant activity (IC50 = 1.1 mM) was exhibited by the [Cu(DFX)(py)] complex.
2-(3-pyridylazo)-3,5-dihydroxybenzoic acid (PAB) and 4-(3-pyridylazo)resorcinol (PAR)
Because of their potential value in this field, we designed, synthesized, and examined the in vitro and in silico antioxidant activities of two azo compounds, 2-(3-pyridylazo)-3,5-dihydroxybenzoic acid (PAB) and 4-(3-pyridylazo)resorcinol (PAR). The generated compounds were studied using 1H-NMR, 13C-NMR, FT-IR, UV-Vis, and mass spectra. The ground-state molecular geometry and vibrational frequencies of the synthesized compounds were determined using DFT simulations at the B3LYP level using the 6-311 G (d,p) basis set. The gauge independent atomic orbital (GIAO) approach was used to determine the chemical shift values of 1H-NMR and 13C-NMR. The HOMO-LUMO calculations were performed using the time-dependent DFT (TD-DFT) approach. The spectra of the compounds produced coincide precisely with those estimated using computational methods. We investigated the antioxidant activities of PAB and PAR using the DPPH assay. PAB molecule antioxidant activity was shown to be greater than that of PAR and the gold standard antioxidant butylated hydroxytoluene (BHT). Furthermore, the antioxidant capacity sequence assessed by the DPPH assay was shown to be reasonably close to the thermodynamic stability features obtained by DFT simulations.