Pyridine as anti-cancerous agent
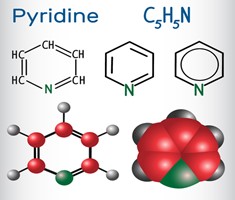
Pyridine is a basic heterocyclic organic compound, with the chemical formula C5H5N. With the exception of one of its C-H groups being exchanged with a nitrogen atom, many of its characteristics are comparable to those of benzene, a well-known and fundamental aromatic molecule. Pyridine's six -electrons, like benzene's, are delocalized across the heterocyclic ring and form a conjugated system. The Huckel aromaticity criteria are fulfilled, and the molecule is flat and two-dimensional.
A Brief History of Pyridine
Pyridine (C5H5N) is an important precursor in the synthesis of various medications and agrochemicals since it is an isomer of benzene. It is also an important reagent and solvent. The term pyridine is derived from the Greek words (pyr) for "fire" and (idine) for aromatic bases. In 1846, Anderson isolated the pyridine backbone from picoline. In 1871, James Dewar and Wilhelm Korner established the molecular structure of pyridine. William Ramsay created a pyridine derivative by combining acetylene and hydrogen cyanide at high temperatures. It is worth noting that pyridine is a nucleoside found in the nucleus of over 7,000 distinct medications now in use.
The Source of Pyridine
The majority of naturally occurring pyridine scaffolds are obtained from plants, including a variety of alkaloids such as the very effective cholinergic drug atropine (Atropa belladonna).

Pyridines: a potent class in the treatment of cancer
Pyrimidines, a member of the pyridinium family of compounds, have shown promising results in the treatment of cancer. Cancer is a broad sickness characterized by uncontrolled and abnormal cell proliferation, which results in tumours that can spread to neighboring tissues. Pyridinium and pyrimidine, two heterocyclic nitrogenous compounds, have various potential applications in cancer therapy. Breast, lymph node, pancreatic, liver, and lung malignancies can all be efficiently treated using compounds produced from synthetic sources.
Pyridine derivatives have anticancer properties
Several diseases, including cancer, have shown remarkable improvement when treated using heterocyclic compounds based on oxygen and nitrogen. Anti-cancer activity is demonstrated by derivatives of the pyrido-fused five-membered heterocyclic ring. The pyridine-fused heterocyclic ring is shared by imidazopyridine, triazolopyridine, pyrrolopyridine, pyrrazolopyridines, thienopyridine, and isoxazolopyridine.
Cancer Treatment with Pyridine Derivatives
- Among brominated products, those having Cu (II) metal complexes with the Schiff base 2-[N-(apicolyl)-amino]-benzophenone on pzridine exhibit the lowest cytotoxicity and the most potential anticancer activity.
- When pyrazoline, pyrimidine, and pyridine molecules with indole activity are synthesized to a high degree of purity, they show significant anticancer effects.
- By substituting the acetyl group in 2-benzoxazolylhydrazon with an acyl group, the inhibitory activities against leukaemia, colon, and ovarian cancer cell lines are considerably enhanced.
- The palladium and zinc complexes of 2-acetyl pyridine thiosemicarbazone have significant anti-tumor activity against human cells while exhibiting little cytotoxicity, making them potential study topics for the creation of novel anti-cancer medications.
- The combination of Cd and 2, 6-diacetyl pyridine has anti-tumor action against the C6 glioma cell line, indicating that it might be effective in the treatment of this kind of brain tumor that is resistant to many current medical therapies.
- When tested against a range of cancer cell lines, the hydrozne of 3- and 4-acetyl pyridine inhibited proliferation at a variety of doses.
- Many pyridine-based ligands exhibit considerable cytotoxicity and have been shown to be antitumor agents when connected with metals.
Metal complexes and their cytotoxicity
- Among metal complexes, those containing Cu (II) have shown very intriguing behavior. It is probable that the aromatic ring's substituent is connected to complex cytotoxicity. The maximum cytotoxicity was revealed to have a high bromine concentration in the aromatic ring.
- Types 30 and 31 cobalt (III) pyridine complexes interact with DNA and efficiently bind the two strands of DNA. Complex 30 outperforms Complex 31 in antitumor effectiveness. When exposed to light at 365 nm, these Co (III) ion complexes may photocleave the plasmid DNA pBR322.
- Benzoyl pyridine thiosemicarbazone-containing derivatives, like quinolones and isoquinolines, are straightforward to synthesize. These medicines exhibit moderate to exceptional cytotoxicity against human cancer cells HuCCA-1, HepG2, A549, and MOLT-3. The antimalarial activity of benzoyl pyridine thiosemicarbazones 32 and 33, as well as a quinoline derivative, 34, ranged from mild to good. 33 of these compounds have been identified as potentially having anticancer and antimalarial effects.
- The N-substituted thiosemicarbazone derivatives of acetyl pyridine and imino pyridine, as well as their gallium (III) and iron (III) complexes have strong anti-cancer activity against the human cancer cell lines 41M and SK-BR3. These compounds' cytotoxicity is mostly determined by the central metal ions; gallium enhances cytotoxicity while iron lessens it.
- The most efficient anti-proliferative medications as iron chelators are 2-acetyl pyridine thiosemicarbazone derivatives.
- Quinol derivatives or their ruthenium or osmium complexes suppress the growth of the human cancer cell lines A549 (non-small cell lung cancer), SW480 (colon-adenoma carcinoma), and CH1 (ovarian carcinoma).
- Copper terpyridinyl complex and 2-acetylpyridine thiosemicarbazone show antiproliferative activity that is superior to the ligand alone. The new organoplatinum (II) 2-acetyl pyridine (III) thiosemicarbazone compound is toxic to human tumor cell lines (HT-29 and Hu Tu-80). The combination has a substantially higher IC50 (anti-proliferation) of 1.2 than the ligand, making it a viable anti-tumor drug.
- Platinum complexes with thiosemicarbazones of 2-acetyl pyridine and 4-acetyl pyridine are being investigated as potential anticancer medicines due to their significant antiproliferative activity against human cells (measured by an IC50 value in M).
- With an IC50 value of 26-90 nM, the Zn combination of 2-acetyl pyridine thiosemicarbazone and a 1-(4-florophenyl)-piprazinyl ring outperforms cis-platin (IC50 = 2- 17 M) against all cell lines.
- The IC50 of the ruthenium II combination with thiosemicarbazone of 2-acetyl pyridine against the ovarian carcinoma cell line 41M is 0.87 M, but the IC50 against the breast cancer cell line SK-BR-3 is 39 M.
- 4-pyridyl anilinothiazol (PAT) is a chemotherapeutic drug used to treat renal cell carcinomas (RCCs) with a novel chemo type that targets tumours activated by the Von Hippel Lindall (VHL) protein.