Pyridine as Antidiabetic
Imidazo [1,2-a]pyridine
Pyridine is a potentially useful bicyclic 5-6 heterocyclic ring that has been designated as a "drug prejudice" scaffold due to its wide range of medicinal chemistry applications such as anticancer, antimycobacterial, antileishmanial, anticonvulsant, antimicrobial, antiviral, antidiabetic, proton pump inhibitor, and insecticidal activities. The studies sheds light on the findings of numerous studies involving imidazo[1,2-a]pyridine scaffold derivatives, which have been shown to yield promising heterocyclic compounds that could be further investigated through the synthesis of new derivatives and the construction of potential drug-like chemical libraries for biological screening in search of new therapeutic agents.
![imidazo[1,2-a]pyridine](https://images.ctfassets.net/aqmu8nx8fse7/22zMPfzoqu5oztnEydezDY/9b9ff61eec5ecab66e327833339b9c2d/imidazo-1-2-a-pyridine.png)
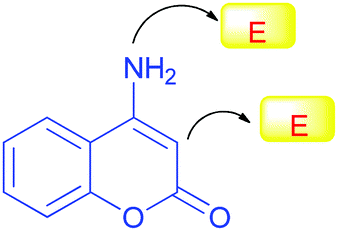
4-aminocoumarins inhibit glucosidase
To test pyridine's efficacy as an anti-diabetic agent, one-pot and three-component synthesis of novel coumarin fused pyridine derivatives were performed. When 4-hydroxycoumarins and ammonium acetate were heated in dichloromethane (DMF), the desired 4-aminocoumarins were formed, which were then cyclized with -azidochalcone derivatives. All of the synthetic compounds tested for their ability to inhibit yeast growth in a petri dish demonstrated significant inhibition of the enzyme -glucosidase.
6-chloro-2-aryl-1H-imidazo [4,5-b]pyridine as anti-diabetic
The synthesis and evaluation of 6-chloro-2-aryl-1H-imidazo [4,5-b]pyridine (1-26) for anti-diabetic, antioxidant, and -glucuronidase inhibitory activity yielded excellent results.
![6-chloro-2-aryl-1H-imidazo [4,5-b]pyridine](https://images.ctfassets.net/aqmu8nx8fse7/7MuJBHvEPdLgaG3vutalgF/486216b790608b02c6c01e239d766c93/6-chloro-2-aryl-1H-imidazo--4_5-b-pyridine.png)
Sulfonamide-based pyridine has alpha-amylase inhibitory activity
Sulfonamides, as a class of organic compounds, have a wide range of biological activities. To create its derivatives with specific biological properties, many different synthetic approaches have been used. The study goes into greater detail about the synthesis of novel pyridine-based heterocyclic compounds with sulfonamide moieties. Their alpha-amylase inhibitory activity was investigated using the obtained sulfonamide-based pyridine scaffold. The structural makeup of newly synthesized compounds was characterized using 1H NMR, 13C NMR, and IR spectroscopy. The grid was created using the results of a sulfonamide molecular docking study against porcine pancreatic alpha-amylase (PDB code: 1LP). All newly synthesized compounds showed anti-diabetic activity.
Glycinium [(pyridine-2, 6-dicarboxylato) oxovanadate (V)] as antidiabetic
The anti-diabetic effects of glycinium [(pyridine-2, 6-dicarboxylato) oxovanadate (V)] complex in a type 2 diabetes were observed in different studies.
![glycinium [(pyridine-2, 6-dicarboxylato) oxovanadate (V)]](https://images.ctfassets.net/aqmu8nx8fse7/4U5oIGMfWt00Ttzif8iTeX/6137d0abc6b5a70a786357ae44ec5246/glycinium_-_pyridine-2__6-dicarboxylato__oxovanadate__V_-.png)
Anti-diabetic activity of thiazolidinedione-based pyridines
Bahekar et al. reported two families of pyridine derivatives, one based on pyrrolo and the other on thieno, in two separate studies. Each of these compounds has been shown to have glucose-dependent insulinotropic activity in vitro against RIN5F cell lines. Kim et al. described the synthesis of some thiazolidinedione-based pyridines and demonstrated their hypoglycemic and hypolipidemic effects.
Thirty newly synthesized thiazole-pyridine derivatives were tested for anti-diabetic activity in Swiss albino mice housed in vivo. These compounds were synthesized by reacting 4,4,7,7-tetra-methyl-4,5,6,7-tetrahydrobenzo[d]thiazol-2-amine with 6-chloronicotinate and then condensing the amines with benzaldehydes. All synthetic compounds reduced glucose levels when compared to the gold standard drug glibenclamide. Potential anti-diabetic drugs with distinct actions have been developed.
Aryl-imidazolines show anti-diabetic activities
The anti-diabetic activities of a series of aryl-imidazolines (2a-l) and aryl-imidazolines (3a-l) designed as analogues of BL 11282 were studied in vitro and in vivo.
Pyrazole-1-carboxylate derivatives as anti-diabetic
The researchers synthesized a wide range of interesting heterocyclic skeletons using the Michael type addition reaction with 1,2; 1,3-bidentate nitrogen and carbon nucleophiles. The cycloaddition of various [alpha],[beta]-unsaturated systems enabled this. pyrazole-1-carboxylate derivatives bromo-3, bromo-5, and bromo-6 (8), pyridinylmethanone (9), nicotinonitrile (10), pyrazolopyridine (11a, 11b), pyran-3-carbonitrile (13), chromenopyridine (14), and N-butyrylpyrazolyl-1-butanone (15). We were able to determine the structures of the synthesized compounds by analyzing their infrared (IR), nuclear magnetic resonance (NMR), and mass spectra. The anti-diabetic potential of a group of newly synthesized compounds was investigated, and two of them, compounds 8 and 11b, showed promising results against [alpha]-glucosidase inhibitors in micro molar concentrations (IC50 values: 13.80–500 [micro]M). Compound 10, on the other hand, had a weaker effect than the gold standard anti-diabetic medication, pyrimidine, nicotinonitrile, pyrazolopyridine, and chromenopyridine.
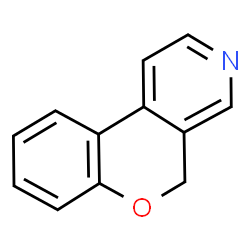
6-Chloro-2-Aryl-1H-imidazo
The spectroscopic methods were utilized to create and analyze 1-26 [4,5-b]pyridine derivatives. Following the completion of docking studies, each derivative was examined for anti-glycation, antioxidant, and -glucuronidase capabilities.
Anti-Diabetic Activity of Pyrrolo[3,4-c]
According to Knutsen et al. 6-methyl-4-substituted pyrrolo [3,4-c] pyridine-1,3(2H)-dione derivatives effectively lower blood sugar levels without affecting insulin levels . The current compounds lower blood glucose levels by increasing glucose uptake into muscle and fat cells. 6-methyl-pyrrolo [3,4-c]pyridine-1,3(2H)-dione derivatives Vitamins 6a-f were found to improve insulin sensitivity. Pyrrolo [3,4-c]pyridine-1,3(2H)-dione derivatives 6 were investigated for their ability to promote glucose incorporation into lipids. The maximum increase in insulin sensitivity achieved in the dose range of 0.3-100 M of the tested compounds was calculated and normalized to the full insulin dose-response (100%). As it turned out, the 4-position phenoxy substituent had a significant impact on the activities of the derivatives. Insulin sensitivity was increased by 7.4-37.4% in mouse adipocytes after treatment with 4-phenoxy-6-methyl-pyrrolo [3,4-c]pyridine-1,3(2H)-dione derivatives (Figure 3). The phenyl ring's para position substituent increased activity. Insulin sensitivity increased by more than 30% when compounds containing 4-phenoxy-(6a), 4-(4-iodophenoxy)-(6b), 4-(3,4-dichlorophenoxy)-(6c), or 4-(4-methylphenoxy)-(6d) were used . The current compounds have been shown to effectively lower blood glucose levels, implying potential use in the treatment and prevention of hyperglycemia and other conditions where blood glucose levels should be reduced. High triglyceride and lipid levels have been linked to diseases such as type 1 diabetes, diabetes caused by obesity, diabetic dyslipidemia, hypertriglyceridemia, insulin resistance, impaired glucose tolerance, hyperlipidemia, cardiovascular disease, and hypertension.
![pyrrolo [3,4-c] pyridine-1,3(2H)-dione](https://images.ctfassets.net/aqmu8nx8fse7/36J0sNG2J0Lbg7ExMQ2vyB/7a77f7b0040b2aaee2b3988dc51226be/pyrrolo_-3_4-c-_pyridine-1_3_2H_-dione.png)
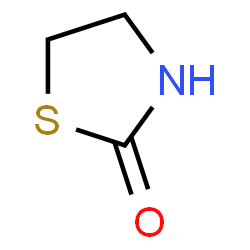
Thiazolidinone derivatives showing anti-diabetic activity
The GOD-POD test reveals that thiazolidinone-containing pyridine derivatives have anti-diabetic effects. Although just a few of these compounds displayed significant anti-diabetic activity, the findings show potential for the future development of a new class of anti-diabetic drugs. Thiazolidinone derivatives have been discovered to have a variety of effects, including hypoglycemic activity. The thiazolidinone ring, a key pharmacophoric component, is responsible for a considerable portion of the antidiabetic activity. As a result, thiazolidinone was chosen as the lead molecule to undergo chemical modification to increase its selectivity, potency, and toxicity. Compounds containing the thiazolidinone ring have been linked to anticonvulsant, antimicrobial, anti-inflammatory, antihistaminic, antihypertensive, hypnotic, and antidiabetic, activities. The existence of heterocyclic rings such as the pyridine ring contributes significantly to the antidiabetic action of several drugs (pioglitazone, rosiglitazone). The inclusion of a pyridine ring and a substituted thiazolidinone ring is frequently required for anti-diabetic activity. It is proposed that certain unique N'-[3-(aryl/alkyl substituted)-4-oxo-1, 3-thiazolidin-2-ylidene] be synthesized in order to generate novel anti-diabetic drugs such as Acetohydrazides (pyridine-2-yloxy).