Pyridine as Antifungal
Anti-fungal properties of pyridine
Fungal infection is a frequent illness that presents a severe expanding threat to mankind involving high morbidity and death every year across the world, especially among immune-compromised individuals. Therefore, it is vital to identify novel antifungal inhibitors, particularly those with unique action mechanisms, with low toxicity and bioavailability. Furthermore, it should be safer and more effective against sensitive and drug-resistant fungus. In recent years, the fused heterocyclic compounds containing bridgehead nitrogen- or oxygen-donor atoms have received tremendous interest due to their biological activities and chemical therapeutic characteristics. Indeed, pyridine and furan derivatives have been lately found to offer pharmacological promise as fungicidal, anti-inflammatory, antiviral, antibacterial, and even anticancer medicines.
Anti-fungal activity of Chloride N-(1-carboxybutyl-4-pyridinium) chitosan
Chloride N-(1-carboxybutyl-4-pyridinium) chitosan was synthesized by adding pyridine moieties to chitosan by nucleophilic substitution (pyridine chitosan) (pyridine chitosan). The antifungal activity of the resulting chitosan derivative was examined by assessing its capacity to inhibit mycelial development and spore germination. According to the findings, the antifungal activity of pyridine chitosan was substantially greater than that of unmodified chitosan. Pyridine chitosan had a minimum inhibitory concentration of 0.13 mg/ml and a minimal fungicidal concentration of 1 mg/ml against Fulvia fulva and 0.13 mg/ml and 4 mg/ml against Botrytis cinerea, respectively. The significant morphological defects seen in pyridine chitosan-treated B. cinerea showed that the chitosan would induce structural damage to the fungal hyphae, thereby stopping the strain from developing. Acute toxicity studies demonstrated that pyridine chitosan was safe for human intake. These findings are relevant for evaluating the usability of this chitosan derivative and for advancing the development of new functional antifungal agents employing chitosan in the food sector.
Tetrahydroimidazo [1,2-a]pyridine hydrazide derivatives against invasive Candidiasis
Recently, the incidence of cases with very invasive candidiasis has climbed by a factor of at least 10 despite the widespread use of new types of medications to treat fungal infections. In addition, antifungal treatment typically fails to cure infections caused by Candida species. The goal of this research was to generate new tetrahydroimidazo [1,2-a]pyridine hydrazide derivatives and assess their cytotoxicity and antifungal activity in vitro. Tetrahydroimidazo [1,2-a] pyridine-2-carboxylic acid hydrazides interacted with various benzaldehydes to form tetrahydroimidazo[1,2-a]pyridine-2-carboxylic acid benzylidene hydrazide derivatives. IR, 1H NMR, MS-FAB+ spectroscopy, and elemental investigations were applied to infer and validate the chemical structures of the compounds. Using agar diffusion and broth microdilution assays, eight novel tetrahydroimidazo[1,2-a]pyridine derivatives were synthesised and tested for antifungal activity against a panel of ten human pathogenic Candida albicans, Candida glabrata, Candida krusei, Candida parapsilosis, Candida tropicalis, Candida utilis, and Candida zeylanoides. In addition, six distinct mammalian cell lines were tested to investigate their cytotoxicity. The analogue 5,6,7,8-tetrahydroimidazo[1,2-a]pyridine-2-carboxylic acid-(4-cyanobenzylidene) displayed the highest inhibitory activity (up to MIC 0.016 mg/mL) against the tested strains of Candida. There was no in vitro toxicity up to 25 g/mL of the same compound, showing that the antifungal activity (MICs 0.016-1 mg/mL) is selective.
![Tetrahydroimidazo [1,2-a]pyridine](https://images.ctfassets.net/aqmu8nx8fse7/1y5FxZl0PlDr6Xlp19m2lJ/82863a4b28357d91363c351cbe854ca6/Tetrahydroimidazo_-1_2-a-pyridine.png)
1,3,4-thiadiazole pyridine possess antifungal properties
Various 1,3,4-thiadiazole compounds with a pyridine group. 1H NMR, MS, and elemental studies all verified the hypothesized structures of 1,3,4- thiadiazoles. The antifungal properties of the compounds in the title were examined. Some of them were demonstrated to have excellent antifungal activities.
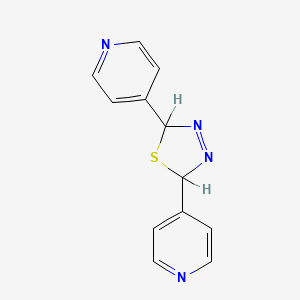
Azaindole amide and pyridine amide against Candidiasis
In vitro antifungal activity of generated quinoline amide, azaindole amide, and pyridine amide was tested. Against Candida albicans and Aspergillus fumigatus, these generated amides demonstrate considerable antifungal effect. Based on our findings, hetero ring amides have the potential to be effective antifungal medicines by inhibiting the GPI production pathway and preventing the Gwt1 protein from executing its purpose.
Picolinic and isonicotinic acid amide against Fusarium culmorum pathogen
Five new complexes of d-electron metal ions with picolinic and isonicotinic acid amide derivatives were produced. All products were characterized in detail with the use of elemental, thermo gravimetric, X-ray diffraction, FT-IR and EPR spectra analysis. The chloride, nitrate or acetate salts of Cu(II), Zn(II), Co(II) and Ni(II) were applied to the synthesis using carboxamide ligands resulting in five novel crystalline compounds. Selected compounds were also employed for antifungal activity evaluation against Fusarium culmorum pathogen. This study was done utilizing two separate methods: the inhibition zone measurements and ocular observation of retardation impact on fungus spores development.
1,2,4-triazolo[4,3-a]pyridines as antifungal
A variety of new 1,2,4-triazolo[4,3-a]pyridines were synthesised, and their structures were studied by 1H NMR, MS, elemental analysis, and single-crystal X-ray diffraction analysis. The antifungal activity was examined. The antifungal activity data suggested that the compound demonstrated excellent activities. Under microwave irradiation conditions, a new family of 1,2,4-triazolo[4,3-a]pyridines was developed and synthesised. The antifungal activity at 100 ppm was tested, and the structures were identified using 1H NMR, MS, and elemental analysis. Some 1,2,4-triazolo[4,3-a]pyridines were shown to have effective antifungal action. Compound was effective against both Fusarium oxysporum and Stemphylium lycopersici (Enjoji) Yamamoto and Cucumebrium species.
![1,2,4-triazolo[4,3-a]pyridines](https://images.ctfassets.net/aqmu8nx8fse7/2NX9RAsLNxrcioXl1pjBry/ce686fd41a1270c169603742bd3ba1ea/1_2_4-triazolo-4_3-a-pyridines.jpg)
Compounds of amide-pyridine against pathogenic fungi
A series of compounds with amide-pyridine scaffolds have been designed and synthesised to treat the rising prevalence of drug-resistant fungal infections based on an analysis of the pharmacophore feature of squalene cyclooxygenase (SE) and 14-demethylase (CYP51) inhibitors and the dual-target active sites. These chemicals were shown to have some antifungal action based on in vitro testing. Compounds 11a and 11b, which showed the best inhibitory effect against drug-resistant pathogenic fungi, with MIC values between 0.125 and 2 g/ml and displayed broad-spectrum antifungal activity. Initial mechanistic research suggested that compound 11b, by blocking SE and CYP51 activity, may have an antifungal effect. Particularly notable chemicals did not exhibit genotoxicity in a plasmid binding experiment. Molecular docking, ADME/T prediction, and a 3D QSAR model development were completed in this work.
Pyridine derivatives against Candida albicans
In order to better understand the antifungal activity of a vast series of novel pyridine derivatives against Candida albicans, quantitative structure-activity relationships (QSAR) experiments were carried out with the help of artificial neural networks (ANNs) (ANNs). Predicting the antibacterial activity of novel pyridine derivatives using structural descriptors derived by computational chemistry is one example of when ANNs have been put to use. Numerous physicochemical and structural features of the pyridine derivatives studied have been linked to their antifungal efficacy against C. albicans. Logarithm of the reciprocal of the minimum inhibitory concentration (log 1/MIC) was used to quantify the activity. Agent molecular descriptors were derived from reference databases for structural fragments and from molecular modeling and quantum chemical computations. When comparing the antifungal activity predicted by ANN, log 1/MICpred, to that obtained from biological studies, log 1/MICexp, for the data utilized in the testing set of pyridine, a correlation coefficient (R) of 0.9112 was achieved.