Pyridine as antimalarial
A study on novel pyridine-containing fosmidomycin showing antimalarial activity
Malaria drug research has centred on blocking the enzyme 1-deoxy-D-xylulose-5-phosphate reductoisomerase (DXR) in the nonmevalonate isoprene biosynthesis pathway. The strong DXR inhibitor fosmidomycin was shown to be both safe and effective in treating Plasmodium falciparum malaria in clinical trials. Based on previous quantitative structure-activity relationship (QSAR) and crystallographic studies, scientists designed, synthesized, and characterized a series of novel pyridine-containing fosmidomycin derivatives with Ki values of 1.9-13 nM that inhibit P. falciparum DXR (PfDXR), with the most potent of these compounds being 11-fold more active than fosmidomycin. These medicines have EC50 values as low as 170 nM for inhibiting the development of multidrug-resistant P. falciparum. The 2.3 crystal structure of PfDXR in complex with one of the inhibitors reveals that the protein's flexible loop undergoes conformational changes upon ligand binding, and that the increased activity is due to a hydrogen bond and favorable hydrophobic interactions between the pyridine group and the PfDXR.
1H-pyrazolo [3,4-b]pyridine 4-aminomethanol against Plasmodium falciparum
The use of pyridine as an antimalarial medicine against drug-resistant Plasmodium falciparum malaria has created a demand for novel antimalarial drugs. By developing and synthesizing 1H-pyrazolo[3,4-b]pyridine 4-aminomethanol compounds as isosteres of the standard quinoline antimalarial mefloquine, new drug candidates based on the conventional ring-bioisosterism notion were generated. The hydrochloride form of these compounds was tested in vitro against chloroquine-sensitive (Sierra Leone D-6) and chloroquine-resistant (Indochina W-2) P. falciparum clones. According to the facts reported here, the 1-H-pyrazolo[3,4-b]pyridine system is a bioisosteric framework to the quinoline system in terms of antimalarial activity.
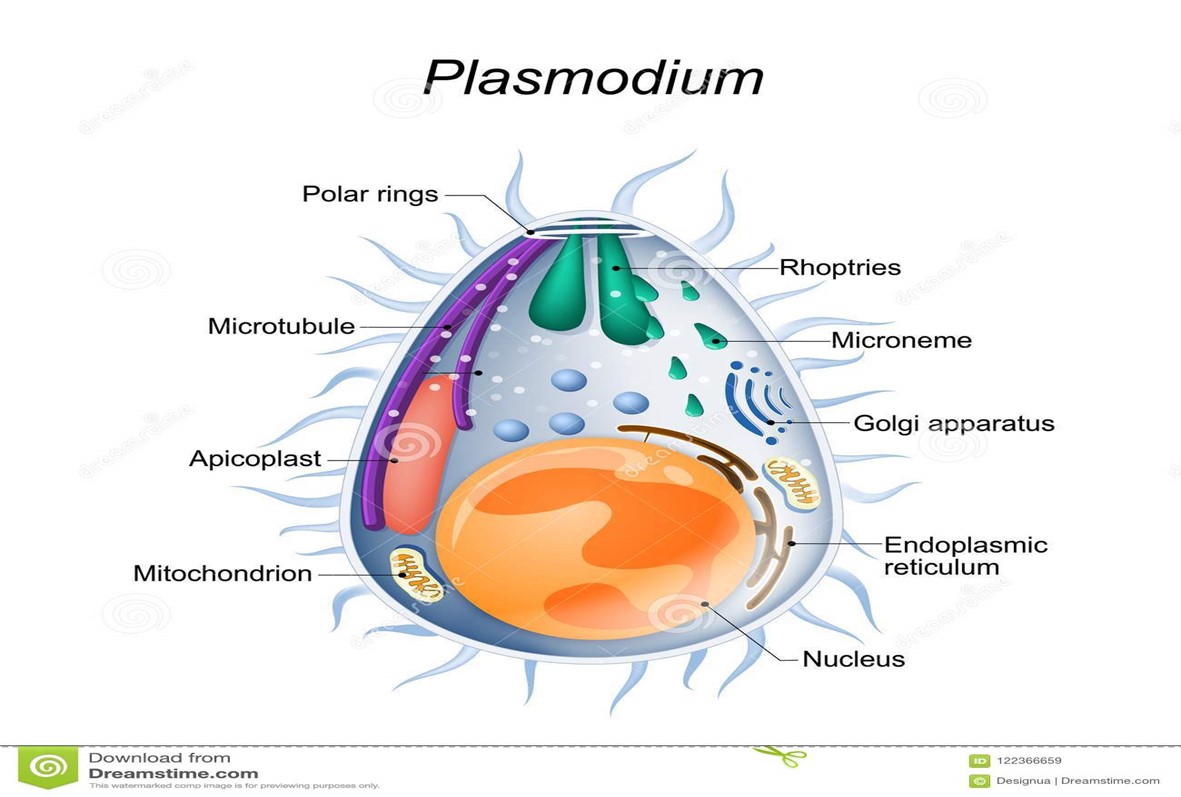
2-(-hydroxyacetyl) pyridine thiosemicarbazones against Plasmodium
A series of 2-(-hydroxyacetyl) pyridine thiosemicarbazones were synthesized in order to develop novel antimalarial and antibacterial medicines. Condensing N4-mono- or N4,N4-disubstituted thiosemicarbazides with 2-(-hydroxyacetyl)pyridine yielded them. The latter was created by selectively oxidizing (2-pyridinyl)-1,2-ethanediol with bromine. The new compounds have a considerable inhibitory effect against Neisseria gonorrhoeae (MIC, 0.5-0.004 g/mL), Neisseria meningitidis (MIC, 0.5-0.032 g/mL), and Staphylococcus aureus (MIC, 0.5-2 g/mL). The majority of these new drugs had strong antimalarial activity in vitro against *Plasmodium falciparum *(Smith strain; ID50, 6.7-38 ng/mL); however, only three of twelve compounds shown modest in vivo effectiveness against Plasmodium berghei. These new chemicals appear to be less toxic to the host and more water-soluble than their 2-acetylpyridine thiosemicarbazone counterparts.
3,5-diaryl-2-aminopyridines showing antiplasmodial activity
By substituting the core pyridine molecule in 3,5-diaryl-2-aminopyridines, a novel family of pyrazine analogues with significant oral antimalarial activity was found. However, antimalarial activity was lost when the pyridine core was further modified, namely by replacing or substituting the 2-amino group. The 3,5-diaryl-2-aminopyrazine series revealed remarkable in vitro metabolic stability in human liver microsomes and significant in vitro antiplasmodial efficacy against Plasmodium falciparum K1 (multidrug resistant) and NF54 (sensitive) strains with nanomolar IC50 values ranging from 6-94 nM.
Pyrazolo [3,4-b]pyridines with anti-Plasmodium falciparum activity
Malaria kills hundreds of thousands of people each year, the majority of whom are children and pregnant women. Scientists demonstrate the isolation and synthesis of a series of pyrazolo [3,4-b]pyridines with anti-Plasmodium falciparum activity, the most deadly species of the malaria parasite. Compounds that were identified as hits demonstrated sub-micromolar in vitro activity against the parasite's intraerythrocytic stage while also being substantially non-toxic to human fibroblast BJ and liver HepG2 cell lines. Furthermore, while our lead compounds are highly efficient against the parasite's liver stage, they are ineffective against the parasite's gametocyte stage. According to the parasitological profiles we have observed thus far, which include analyses of mortality rates, docking, and molecular dynamics, our compounds may target the Qo binding site of cytochrome bc1.
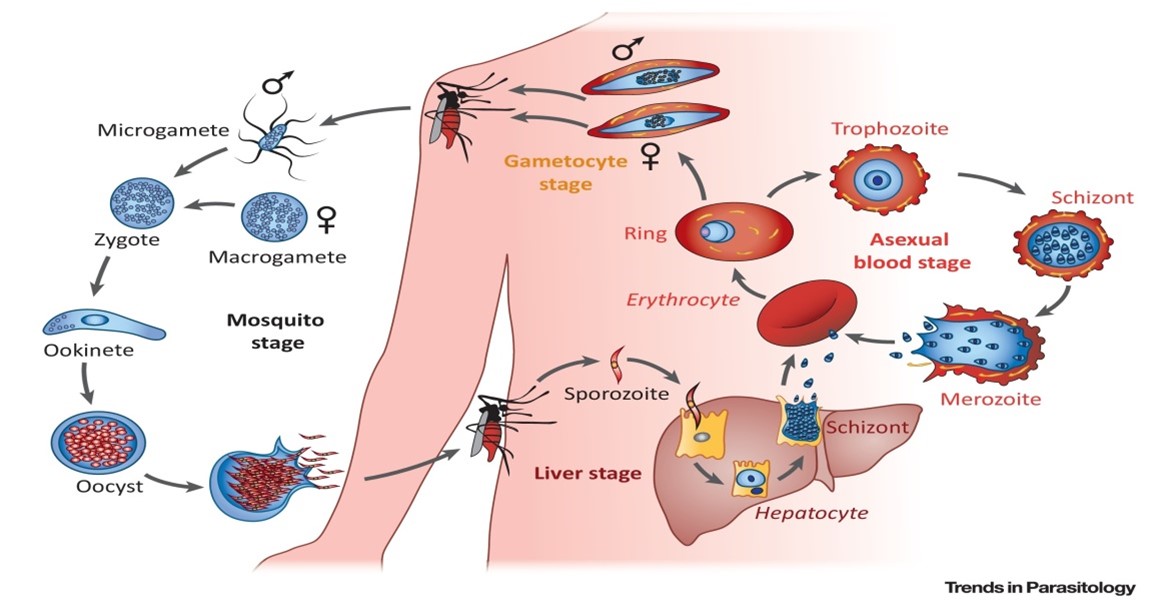
Pyridine quinoline hybrid molecule as antimalarial
A new pyridine quinoline hybrid molecule was synthesized and evaluated for antimalarial effectiveness against a chloroquine-susceptible strain of Plasmodium falciparum as well as as a -hematin biosynthesis inhibitor. Three synthesized pyridine-quinoline hybrid compounds and one bisquinoline molecule were studied for their antimalarial and haem polymerization inhibition (HPIA) characteristics. The compounds were determined to be good inhibitors of haem polymerization but had very little antimalarial activity. This was because they had a lower vacuole accumulation ratio (VAR) than chloroquine. Such chemicals can be used to produce new antimalarial that target the haem detoxification pathway in the malaria parasite.
4-phenylthieno [2,3-b]pyridines inhibit PfGSK-3
Malaria is one of the most frequent infectious diseases in tropical regions. Reports of artemisinin resistance emphasize the critical need for novel anti-malarial drugs that are structurally unique from artemisinins and target a different biological target. The plasmodial glycogen synthase kinase-3 protein is a new target for anti-malarial medicines (PfGSK-3). There is evidence that 4-phenylthieno [2,3-b]pyridines inhibit PfGSK-3 and so have antiplasmodial activity. Furthermore, the role of axial chirality within the chemical class in antiplasmodial activity and PfGSK-3 inhibition was investigated. The para position on the 4-phenyl ring of the aforementioned compounds was discovered to be a suitable location for connecting side chains. Alkylamino groups retained antiparasitic action; however alkoxy substituents at this location reduced antiplasmodial activity. The addition of alkylamino side chains to selective PfGSK-inhibitors of the 4-phenylthieno [2,3-b]pyridine family improves their antiplasmodial activity and makes them more water soluble. The axial chirality of these chemicals has a significant impact on biological activity.
Pyridine against chloroquine-resistant strains
Compounds based on pyridine have antimalarial effects and are very effective against chloroquine-resistant strains due to hydrogen-bond interactions between the pyridine nitrogen and the cysteine of target proteins in the pathogen, making them a promising candidate for the development of antimalarial drugs.
Copper (II) complexes containing pyridine-2-carboxamidrazone ligands as antimalarial
The authors discuss the synthesis and characterization of novel copper (II) complexes containing pyridine-2-carboxamidrazone ligands. The in vitro antimalarial activity of the ligands and their copper complexes underlines the importance of metal conjugation in the creation of an effective antimalarial medication.
4-isonicotinic hydrazide acts as antimalarial
4-isonicotinic hydrazide, a popular TB treatment, inhibits enoyl-ACP reductase, a crucial enzyme in the synthesis of fatty acids. As a result, pyridine analogues may hinder P. falciparum from generating the necessary fatty acids required for survival in the host. Compounds having several target site activities have a higher chance of becoming functional.
Ugi-Smiles couplings with 4-hydroxy and mercapto pyridines as antimalarial
Ugi-Smiles couplings with 4-hydroxy and mercapto pyridines were effective. Antimalarial drugs may be synthesized quickly and easily using multicomponent processes on quinoline derivatives.