Pyridine as a ligand
Despite being the simplest six-membered heterocyclic, the structure of pyridine (C5 H5 N) is quite close to that of benzene. The strong electronegativity of the nitrogen in the benzene ring impacts the resonance environment in a totally different way than the carbon version's chemistry. Because of the presence of nitrogen and its lone pair in an aromatic environment, pyridine is unique in chemistry. When the "N" atom's sp2 lone-pair orbital faces away from the ring structure and overlaps with an empty metal orbital, a bonding contact is formed. Pyridine serves as a ligand in this approach, and it has been utilized to create metal complexes with every transition metal. Pyridine and its derivatives have created a vast body of literature on metal complexes as a result of their frequent use. Many complexes of scholarly and industrial importance have been synthesized.
Chiral versions of pyridines
Despite the extensive usage of pyridine derivatives as supporting ligands in catalysis, the development of chiral versions of these molecules has been slow.
Determination of Copper (II) complexes of pyridine-based ligands
Copper (II) complexes of pyridine-based ligands functionalized with alanine (PydiAla) and tyrosine (PydiTyr) moieties were formed to construct novel superoxide dismutase mimics. PH-potentiometric, spectroscopic (UV-vis, circular dichroism, mass spectrometry, electron paramagnetic resonance spectroscopy), computational (DFT), and X-ray diffraction techniques were employed to determine the characteristics of the complexes. Because of the (Npy, N-, N-) donor set and the binding of the carboxylate pendant arms, copper (II) complexes of both ligands are extremely stable. Despite sharing the same coordination mode, the tyrosine-containing system shows a greater affinity for binding copper (II), which could be attributed to the aromatic moiety of the side chains. Both copper (II) complexes can bind N-methyl imidazole, and the formation of the corresponding ternary species was seen at physiological pH. The binary and ternary copper (II) complexes have high SOD activity. The PydiTyr complex has 10 times the activity of the PydiAla complex. The former species' phenolic OH group is most likely to blame for enabling the binding of the superoxide anion radical to the metal core, making this species more reactive. The discoveries pave the path for the creation of highly effective copper (II) mimics with potential medical and industrial applications.
Pyridine is a good ligand for transition metals
Pyridine is a good ligand for transition metals and can form metal complexes with a wide range of elements due to its Lewis basic nature, which arises in its nitrogen lone pair. It is typically a weak monodentate ligand that can bind metal at various concentrations to form a wide range of metal complexes. Over time, a large body of study on transition metal complexes coordinated by pyridine has accumulated. Pyridine and its various derivatives have been explored by inorganic chemists in order to develop and manufacture a wide range of metal complexes. Polypyridine systems have been examined for their possible utility in the ongoing design of pyridine ligands, in which two or more pyridine moieties are joined to produce chelating multidentate ligands. Bipyridine, a molecule composed of two linked pyridine rings, is used in transition metal chemistry. The bright ruthenium bipyridine complexes and their peculiar photochemistry are an example of bipyridine metal chemistry. For a long time, transition metal chemists have been intrigued by phenanthroline, terpyridine, and other multidentate ligands.
Ligating power of Pyridine
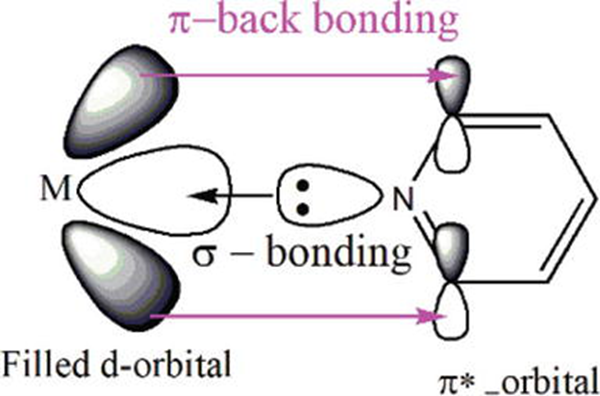
The spectrochemical ligand series of crystal field theory (CFT) illustrate ligand organization in relation to metal d-orbital splitting ability, and pyridine is demonstrated to be a moderately potent ligand. This can be regarded as evidence of strong electrostatic interactions between pyridine lone pairs and metal d-orbitals. Despite its neutrality, pyridine produces a moderately substantial d-orbital splitting, indicating a strong bonding relationship to metal centres. The pyridine sp2 lone pair orbital overlaps with hybridized metal orbitals, which is taken into consideration by both the valence bond theory (VBT) and the crystal field theory (CFT). In addition to interacting with nitrogen lone pair orbitals, the ring -electron can connect with metal ions. Metal electron density can also be accepted by delocalized * anti-bonding orbitals (Figure 3). The pyridine can also engage in hydrogen bonding and - stacking-like weak interactions. As a result, when interacting with metal ions, pyridine has additional bonding orbitals to use.
Examples of Pyridine complexes with transition metals
There is a variety of material about pyridine transition metal complexes accessible. Pyridine was revealed to be capable of coordinating any transition metal, resulting in a diverse range of metal complexes with various oxidation states. Despite efforts to incorporate an increasing number of pyridines in the metal coordination sphere, exclusive pyridine complexes such as [M(py)4]n+ or [M(py)6]n+ (where M = transition metal) are rare. Metal complexes with pyridine and its derivatives that can act as bidentate or tridentate ligands are an important aspect of metal-pyridine chemistry.
Scandium and yttrium
Scandium and yttrium both prefer four-coordinated structures containing three pyridine units. The coordination number may be influenced by indicators of binding ligand characteristics. In addition, pyridine and thiocyanate (SCN) ligands are utilised to form five and six coordinated complexes, respectively. Picolinic acid, a pyridine derivative that can act as a bidentate ligand, for example, produces complexes with a higher coordination number. There are numerous complexes known that involve various forms of substituted pyridines. In the +1 and +3 states, Sc and Y are known to form these complexes. Pyridine coordinated complexes include [Sc(py)3Cl], [Y(py)3Cl], [Sc(py)2(NCS)3], and [Sc(py)3(NCS)3]. The metal salt and pyridine could be employed to create the complexes at room temperature.
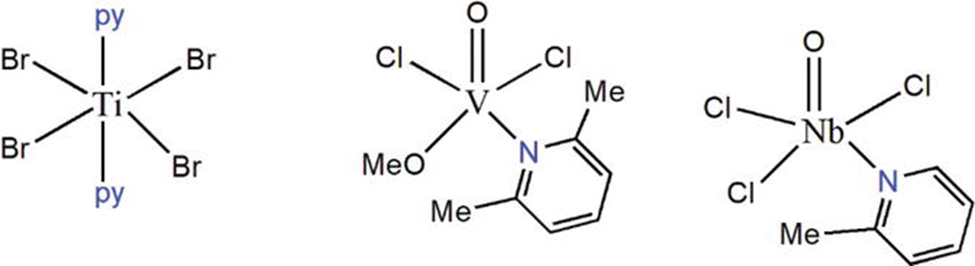
Titanium pyridine complexes
Titanium pyridine complexes are most abundant in Ti (IV) valence states, however they can also be found in other valence states (II-IV). Both the zirconium states Zr(III) and Zr(IV) can produce pyridine complexes. Titanium and zirconium +4 pyridine complexes are the most common. Complexes of Ti(II) and Ti(IV) pyridines ([Ti(py)2(Cl)4], [Ti(py)2(Br)4]) may develop in the presence of halogen ligands. Ti(II) complexes, for example, are tetra-coordinated, but Ti(III) and Ti(V) complexes are hexa- and octa-coordinated, respectively [10]. The behavior of Zr(IV) pyridine complexes is similar to that of titanium. The well-known complex [Zr (py)2Cl4] [11] is a well-known complex. Three such complexes are depicted in the figure below.