Pyridine as pesticide
The entire pyridine compound synthesis process may be seen as a tree, with the pyridine base serving as the "root." Several goods can be manufactured farther down the production line using pyridine intermediates.
Pyridine: a necessary intermediate
Pyridine is a necessary intermediate in the manufacture of several medicines, pesticides, and even veterinary remedies. Insecticides account for up to half of total output, and there are over 30 distinct products that may be manufactured from them.
Development of pyridine-containing pesticides
Pesticides are effective, safe, and long-lasting because of the pyridine moiety. They are ecologically sustainable while meeting the demands and trends of pesticide development. The rapid development of pyridine-containing pesticides throughout the world is one of the most visible developments in the pesticide business.
Methyl pyridine molecules in agrochemical sector
Early on, it was revealed that methyl pyridine molecules, namely a combination of 3-methylpyridine, 2-methylpyridine, 4-methylpyridine, and 2-chloro-5-chloromethylpyridine, accounted for more than 20% of pyridine byproducts (CCMP). Humans set out to research and refine these methyl pyridine compounds in order to advance the pyridine business. After years of study and development, the agrochemical sector has discovered significant use for methyl pyridine derivatives, notably 3-methylpyridine.
Examples of pyridine pesticides
- The present pesticide generation is the fourth since the industry began creating them.
- DDT and benzene are examples of the first generation of pesticides; methomyl and parathion are examples of the second generation; cypermethrin and deltamethrin are examples of the third generation;
- and pyridine pesticides such as haloxyfop-P-methyl, fluazinam, fluopyram, and picoxystrobin are examples of the fourth generation.
- The majority of the intermediates in the most recent generation of sophisticated pesticides are fluorine-containing pyridine compounds, with methylpyridine derivatives serving as the key raw material in the production of these highly effective and generally safe agrochemicals.
- Popular methyl pyridines include two-chloro-5-chloromethylpyridine (CCMP), two-methylpyridine (DMP), three-methylpyridine (3-MP), and four-methylpyridine (4-MP). The agrochemical industry values methylpyridine compounds and these four are the finest of the group.
- Several pyridine compounds can be formed depending on the starting aldehyde and the reaction conditions. Acetaldehyde and ammonia combine to yield pyridine, 2-methylpyridine, and 4-methylpyridine; acetaldehyde and formaldehyde react with ammonia to form pyridine, 3-methylpyridine, and so on. To make CCMP, either 3-methylpyridine or dicyclopentadiene (the starting material) can be treated with acryl aldehyde or acrylonitrile.
- The skeleton of trifluoromethyl pyridine has proved critical in the creation of novel pesticide compounds, and it is found in around 27 distinct commercial pesticides. Five of them are used to control insects. Pyridine-based chemicals are widely used in agriculture as fungicides, insecticides/acaricides, herbicides, and other pesticides.
- The creation of novel fluorine-containing pesticides has lately become a hot topic.
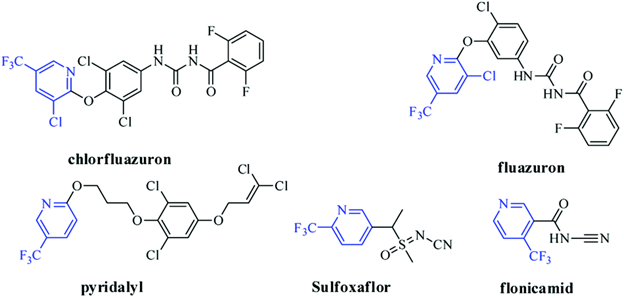
Methylpyridine chlorinated and fluorinated analogues
A large range of useful chemicals can be obtained by chlorinating or fluorinating methylpyridine. The addition of trichloro or trifluoro to the pyridine moiety at positions 2-6; chloro, fluoro, amino, hydroxyl, bromo, or iodo to position 2; and chlorio, fluoro, amino, or hydroxyl at positions 2 and 3 are all legitimate techniques of derivation. 2-trifluoromethyl pyridine, 2-chloro-3-trifluoromethyl pyridine, 2-chloro-4-trichloromethyl pyridine, 2-amino-5-trifluoromethyl pyridine, 2-amino-3-chloro-5-trifluoromethyl pyridine, 2-chloro-6-trifluoromethyl pyridine, and others are examples of products.
The fluorine-containing pyridine as pesticides
The key route for future expansion for methylpyridine is still through pesticide products. Following fluorination, the biological activity of pyridine heterocyclic compounds is boosted by a factor of five; this is done at a lower consumption rate and with fewer lingering residues in the soil, meeting ever-stricter environmental criteria. As a result, the fluorine-containing pyridine insecticides generated downstream have emerged as the backbone of the present generation of pest-control compounds. According to incomplete statistics, fluorine-containing compounds are responsible for more than half of the new insecticides developed in the last decade.
Categories of Pesticides
The compounds that will be utilized in future insecticides may be divided into three categories:
- Pesticides containing a pyridine moiety, such as the aforementioned pyridine downstream products, such as haloxyfop-P-methyl, fluazifop-P-butyl, fluazinam, and others, have high activity and low toxicity.
- Single-activity chiral compounds, such as quizalofop-P-ethyl and gibberellins (in which the ineffective component is changed into the effective part, to prevent the ineffectual or antagonistic impact of isomers)
- Third, biologically derived pesticides that are non-toxic, environmentally friendly, and safe to use near non-target plants and animals. Common products include spinosad, glufosinate-P, and others.
Biodegradation of pyridine derivatives
Although pyridine derivatives are not commonly considered xenobiotic, they are an important class of aromatic compounds that are predominantly produced by human activities. Pyridine derivatization enables the creation of a variety of medicinal and agricultural chemical derivatives. Despite their structural similarity to phenolic compounds, pyridines have characteristics that distinguish them from homocyclic compounds, which may have important ramifications for biodegradation. The presence of nitrogen in the ring determines the reactivity of pyridine derivatives. Several common threads concerning the biodegradation of pyridine derivatives have emerged after 60 years of research, but new results are continuously challenging our existing understanding of this process.
Thioether ligands (Q1-Q4) pesticide
Four distinct thioether ligands (Q1-Q4) have been proven to be capable of producing twenty-one different transition metal complexes; two more complexes have also been generated and tested. Scientists were able to establish some plausible rules based on variations in metal ions, anions, and spatial structures by comparing the pesticide activities of four distinct ligands and their 21 different transition metal complexes. The findings revealed that complexes containing the Ag+ ion, which may incorporate a bimetallic ring as a structural unit, have exceptionally powerful pesticide properties.
Pyridoxine Carboxamide-based pesticides
If the substituent R is as specified in claim 1, the formula (I) is useful as a pesticide. The invention focuses on 3-pyridylcarboxamide derivatives with the following formula (I).
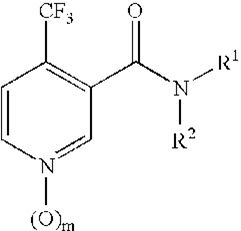
Wherein the various symbols signify what they say in the description, compositions thereof, methods of employing same to manage pests, and processes of producing same.
Adverse effects of Pyridine
- Pyridine, an N-heterocyclic compound, is widely used as a solvent in the dye, explosive, pharmaceutical, pesticide, and agricultural industries. The dangers of pyridine in these industries' effluent on human health are well known.
- Water containing formaldehyde is produced by resin manufacturers and petrochemical plants.
- Formaldehyde is a common active component in preservatives and disinfectants. If they are discarded after use, their antibacterial qualities may present issues for wastewater treatment systems.
- Following the discovery of formaldehyde's carcinogenicity in experimental animals, there is now even more cause to be concerned that it poses a carcinogenic risk to humans.
- Commercial solvent, resin, and pesticide manufacturing wastewater all contain potentially carcinogenic compounds such as pyridine and formaldehyde. The toxicity of pyridine and formaldehyde on marine life is unknown.