Pyridines deep dive: Properties, structure, synthesis and sources
Pyridine is an Azaarene, having a benzene core in which one -CH group is replaced by a nitrogen atom. It is the parent compound of the class pyridines It has a role in containment of the surrounding environment. In addition to having an azaarene and a pyridine as its organic heteromonocyclic parents, it is also a monocyclic heteroarene.
Properties
Pyridine is a liquid that is transparent, colorless, or very light yellow in appearance, and it has a pungent, disagreeable odor. It has a density of 0.978 g/cm3. The temperature at which an explosion can occur is 68 degrees Fahrenheit. Its vapors are heavier than air. If inhaled or swallowed, this substance is extremely hazardous. During the combustion process, harmful oxides of nitrogen are created.
The temperature at which this substance starts to melt is -44 degrees Fahrenheit, whereas the point at which it starts to boil is 239 degrees Fahrenheit at 760 millimeters of mercury. It is possible to mix it with water, alcohol, ether, petroleum ether, oils, and a broad range of other organic liquids when the temperature is at room temperature, which is 20 degrees Celsius.
Structure
Pyridine has a molecular weight of 79.102 g/mol and the chemical formula C5H5N. The chemical structure of it may be described using the following diagram:
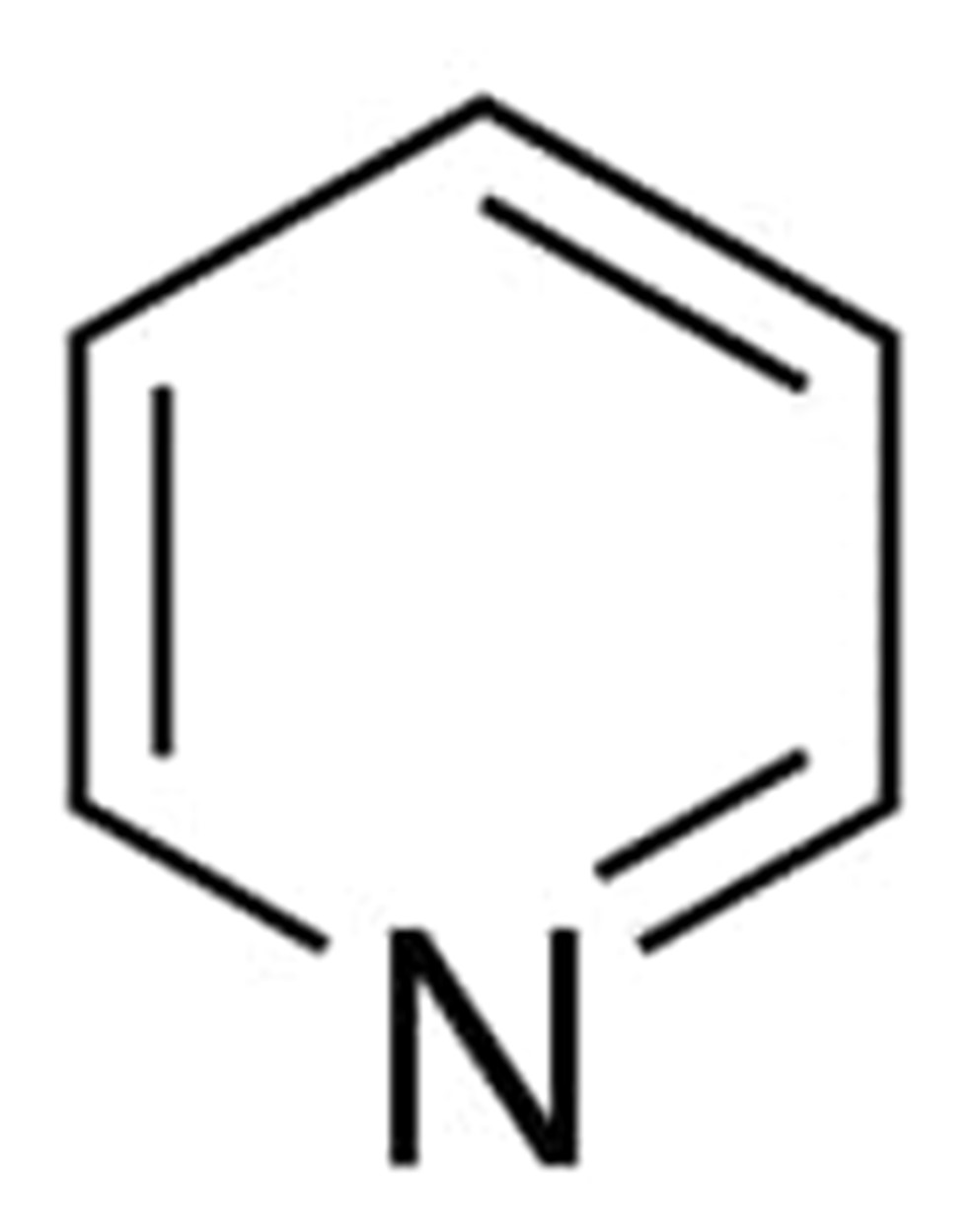
Benzene and pyridine have identical electronic structures; yet, pyridine possesses a number of properties that set it apart from benzene. Pyridine is completely miscible with water, in contrast to benzene, which only has a very low solubility in water. In the acid-base sense, benzene, like other hydrocarbons, is neutral, but because to the inclusion of a nitrogen atom, pyridine is a very weak base.
In the Hantzsch Widman nomenclature, which is preferred by IUPAC, pyridine is referred to as azinine. Common names are typically used in place of systematic names for identifying heterocyclic compounds due to the fact that systematic names are only used for simple compounds very infrequently. It is not recommended to utilize azinine or azine as a substitute for pyridine, as stated by the International Union of Pure and Applied Chemistry (IUPAC). The nitrogen is placed at the beginning of the numbering sequence for each of the ring atoms in pyridine.
In some instances, the names of the sites are notated using the a-z notation of the Greek alphabet, whereas in other instances, the nomenclature for homoaromatic substitution patterns (ortho, meta, and para) is used. In this instance, the locations 2, 3, and 4 are denoted by the letters ortho, meta, and para, respectively. Pyridine derivatives are referred to as pyridinyl in the chemical nomenclature, and the position of the substituted atom is denoted by the prefix. Despite the fact that the systematic nomenclature is preferred by the IUPAC, pyridyl is frequently used instead. Pyridinium is the name given to the cationic derivative that is produced when an electrophile is introduced to an atom of nitrogen.
Synthesis
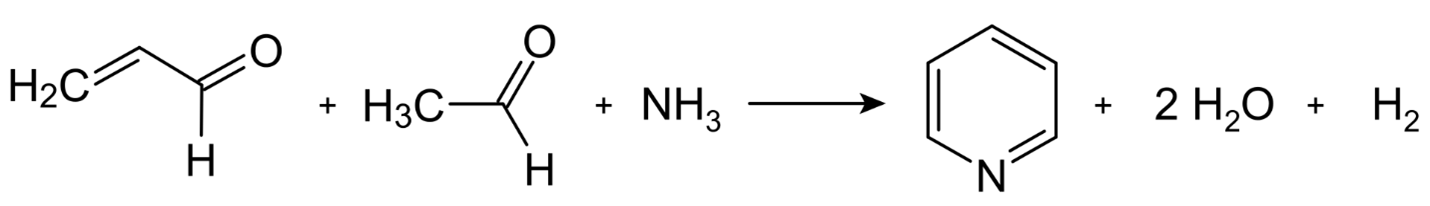
In the year 1881, the German university of Leipzig was the location where scientist Arthur Rudolf Hantzsch made the painstaking and low-yield discovery that led to the synthesis of pyridine. In 1924, the Russian chemist Aleksei Chichibabin improved upon this process by using formaldehyde, acetaldehyde, and ammonia to produce dihydropyridine. This dihydropyridine was then oxidized to pyridine at high temperatures using a transition metal fluoride catalyst. Chichibabin's innovation was published in the journal Chemical and Pharmaceutical Bulletin. In addition to this method, other strategies are utilized, such as the oxidative dealkylation of alkylpyridines.
In 1846, Anderson extracted pyridine from bone pyrolyzates and coal tar, two sources from which it had been previously shown that it existed. In the process of preparing industrial products, tetrahydrofurfuryl alcohol and ammonia are now utilized. Examples of pyridines include nicotine, which may be found in cigarettes, sulfapyridine, which is used as an antibiotic, and pyridoxine, which is vitamin B6 and acts as a benzene cofactor.
The Chichibabin pyridine synthesis was the first publication of the fundamental concept that is used in several industrial procedures today. This principle was discovered in 1924.
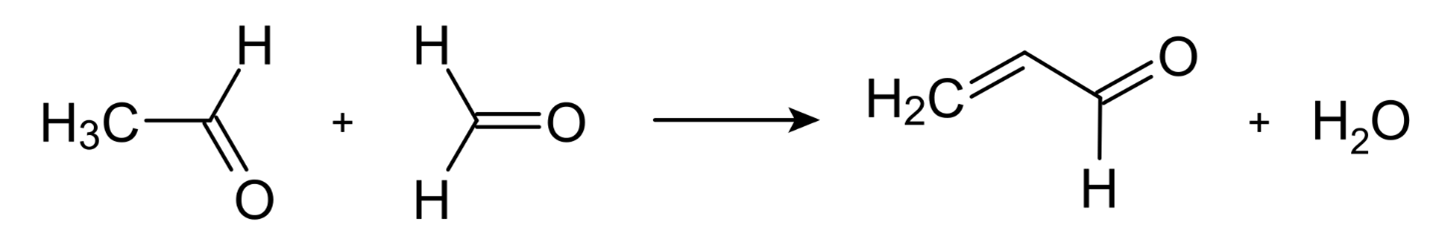
This reaction may be thought of as having a broad mechanism that involves the condensation of aldehydes, ketones, or,-unsaturated carbonyl compounds, or mixes of these, in ammonia or ammonia derivatives. Although the precursors are very inexpensive, the Chichibabin pyridine synthesis often results in low yields of around 30 percent. Formaldehyde and acetaldehyde are the starting materials for the synthesis of unsubstituted pyridine, for example.
Acrolein is the first product formed when acetaldehyde and formaldehyde undergo Knoevenagel condensation. Acrinaldehyde and ammonia undergo a reaction that results in the formation of dihydropyridine, which is then converted to pyridine by oxidation. This process takes place in a gaseous form at temperatures ranging from 400 to 450 degrees Celsius, and the temperature range covers the whole time it is being carried out. The typical catalysts are alumina that has been modified and silica. Because of the changes that have been made to the method, it is now possible to produce a variety of methylpyridines.
Sources
Marshmallows (Althaea officinalis) and the leaves and roots of the belladonna plant (Atropa belladonna) are the only naturally occurring sources of the chemical pyridine (Althaea officinalis).
On the other hand, pyridine derivatives may be found rather commonly in biomolecules such as alkaloids.
As a result of the roasting and canning processes, which generate volatile organic compounds, common meals such as fried chicken, sukiyaki, roasted coffee, potato chips, and fried bacon contain trace amounts of pyridine. Other examples of these types of foods include.
Detectable levels of pyridine may be found in a variety of foods and drinks, including Beaufort cheese, vaginal fluids, gingivitis sufferers' saliva, black tea, and sunflower honey.