Bromide & Bromide Derivatives Available For Purchase
At Chempanda, we offer a range of bromide and bromide derivative chemical compounds that can be shipped to anywhere across the globe. We offer over 18000 different compounds that are available in varying purities and quantities.
Our Bromide Suppliers
We have partnered with a range of chemical manufacturers who specialize exclusively in synthesizing bromides and their derivatives. With years of industry experience, our suppliers offer the most common bromide compounds, or can custom manufacture bromide compounds that you cannot purchase from most chemical suppliers.
With all orders, you can order the exact amount of chemical that you need - with no minimum order - in a cost and time effective manner that other suppliers cannot match.
Bromide Physical Properties & Structure
Bromides refer to any molecule containing one or more of the halogen atoms, bromine. Due to the outer orbital of bromine containing seven electrons, it readily forms negatively charged ions which make it highly reactive and easy to form bromides.
The physical and chemical properties of bromides vary as they are determined by the reactions and formations they make with other chemical groups.
Bromide Derivatives
The classification of bromide compounds is broad due to the way that a single bromine atom is enough for a compound to be classified as a bromide.
When referring to bromide derivatives, this can mean any compound containing bromine, but most commonly referring to simple bromides such as zinc bromide (ZnBr) or calcium bromide (CaBr) for example.
The Reactivity of Bromides
As a halogen element, bromine is highly reactive and does not occur as freely in nature. With metals, bromine is reactive and can form vigorous reactions with some metals.
Bromides that react with alkanes are known as a class of haloalkanes, which can form with one or more halogen atoms should reaction conditions allow it. Bromides also react with aryls to form aryl halides.
Applications of Bromides & Derivatives
The bromide and derivatives market is estimated to be worth around $1 billion (US), due to the fact that bromides are used widely in the oil and gas drilling industry, in addition to pharmaceuticals and the energy sector. Bromides are used as flame retardants, and in the energy and battery sector for industrial and commercial applications.
Related Article(s)
Bromophenols: types and applicationsJun 4, 2023
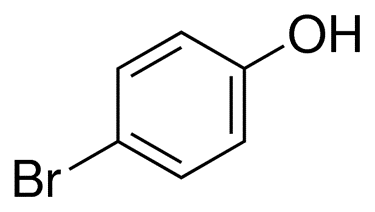
Bromophenols are the products obtained from the halogenation of phenol by electrophilic bromine. It is a powder that can range in colour from tan to orange, and it is used as an indicator of acid and base.
Bromides: History, sources, types, applications and hazards Aug 10, 2022

Bromine is located in the periodic table's halogens group, and its negatively charged form (Br) is an ion known as a bromide ion. Bromides that are colourless and have a wide range of uses, including anticonvulsants, flame retardants, and cell stains.
Methylphenol: Common isomers, structure, synthesis, applications and natural sourcesJun 28, 2022
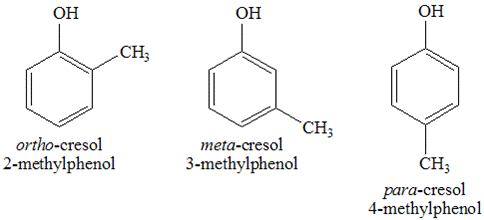
Methylphenol is an aromatic compound with a chemical structure represented by the formula CH3C6H4(OH). Its molar mass is 108.140 gmol and these compounds exist in both liquid and solid state.
Bromopyridine: Common isomorphs, synthesis, applications and storageJun 4, 2022
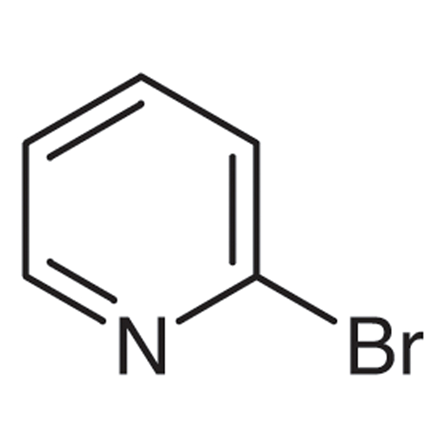
Bromopyridine is an organic compound with the formula BrC5H4N, molar mass 158.00 g/mol with a boiling point192°C to 194°C. It is a colorless liquid that is used as an intermediate in organic synthesis.