13019-32-4
7-Bromoquinolin-8-ol
- 7-bromoquinolin-8-ol
- 13019-32-4
- 7-Bromo-8-hydroxyquinoline
- 8-quinolinol, 7-bromo-
- 7-Bromo-8-quinolinol
Get a free no-obligation quote
We typically respond within 30 minutes during business hours!
Related Article(s)
Bromophenols: types and applicationsJun 4, 2023
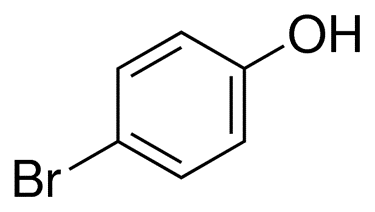
Bromophenols are the products obtained from the halogenation of phenol by electrophilic bromine. It is a powder that can range in colour from tan to orange, and it is used as an indicator of acid and base.
Bromides: History, sources, types, applications and hazards Aug 10, 2022

Bromine is located in the periodic table's halogens group, and its negatively charged form (Br) is an ion known as a bromide ion. Bromides that are colourless and have a wide range of uses, including anticonvulsants, flame retardants, and cell stains.
Alcohols: Classification, sources, commercially significant types and usesJul 31, 2022
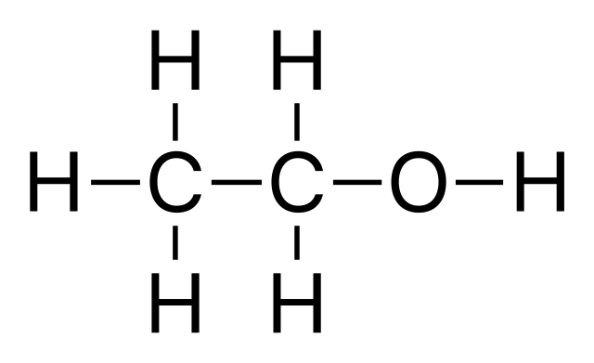
Alcohols are chemical substances that include an aliphatic carbon atom with a hydroxyl (OH) functional group attached. Because all alcohols share OH, we usually write alcohols as the general formula ROH, where R is an alkyl group.
Methylphenol: Common isomers, structure, synthesis, applications and natural sourcesJun 28, 2022
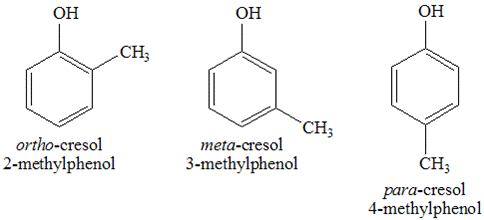
Methylphenol is an aromatic compound with a chemical structure represented by the formula CH3C6H4(OH). Its molar mass is 108.140 gmol and these compounds exist in both liquid and solid state.

