- 2-Amino-6-bromo-4-chlorophenol
- 179314-60-4
- SCHEMBL3355916
- DTXSID70573594
- 2-amino-6-bromo-4-chloro-phenol
Get a free no-obligation quote
We typically respond within 30 minutes during business hours!
Related Article(s)
Chloromethyl: compounds, synthesis and safetyJun 11, 2023
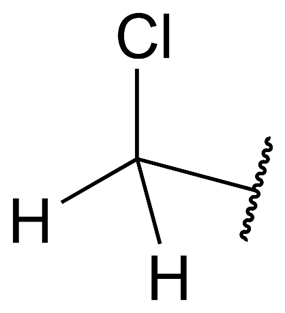
In the field of organic chemistry, a functional group known as the chloromethyl group (CH2Cl) can be found. In the formula for the methyl group (CH3), one hydrogen atom was replaced with a chlorine atom, which resulted in the creation of a new chemical entity that was subsequently given a new name.
Bromophenols: types and applicationsJun 4, 2023
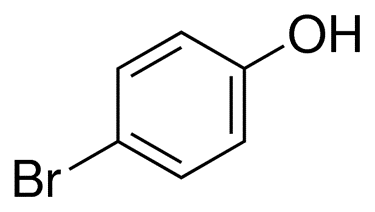
Bromophenols are the products obtained from the halogenation of phenol by electrophilic bromine. It is a powder that can range in colour from tan to orange, and it is used as an indicator of acid and base.
Bromides: History, sources, types, applications and hazards Aug 10, 2022

Bromine is located in the periodic table's halogens group, and its negatively charged form (Br) is an ion known as a bromide ion. Bromides that are colourless and have a wide range of uses, including anticonvulsants, flame retardants, and cell stains.
Chlorides: Structure, primary types and applicationsAug 5, 2022

Chlorides are the negatively charged ions formed by chlorine (Cl-). To this end, chlorides are widely defined as any material containing chlorine. This category includes chlorine salts and acids such as hydrochloric acid.
Aryls: Structure, nomenclature, types and applicationsAug 3, 2022
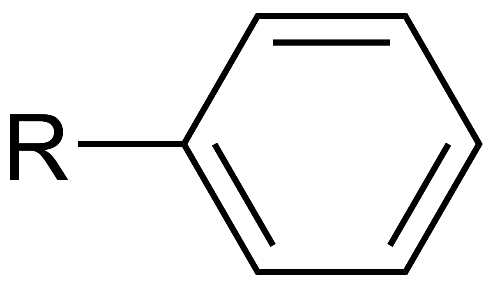
An aryl group is the name given to the functional group that is produced when an aromatic ring complex is reduced to its simplest form by removing one hydrogen atom. The aromatic ring will almost always be constituted by a hydrocarbon in most instances.
