21402-26-6
4-Bromo-3-chloroaniline
- 4-Bromo-3-chloroaniline
- 21402-26-6
- Benzenamine, 4-bromo-3-chloro-
- 3-CHLORO-4-BROMOANILINE
- UNII-2GTM4SW4OH
Get a free no-obligation quote
We typically respond within 30 minutes during business hours!
Related Article(s)
Iodobenzene: Synthesis, reactions, environmental exposure, safety and applicationsJun 25, 2023
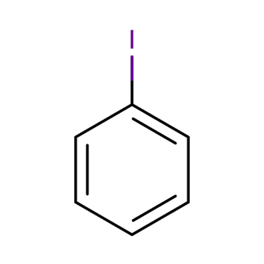
Iodobenzene is an organoiodine molecule that has one of its benzene rings switched out for an iodine atom. Iodobenzene has an empirical formula of C6H5I and a molecular weight of 204.01g/mol. In organic chemistry, it is utilised as an important intermediate in the synthesis process.
Methoxy benzene: synthesis and applicationsJun 18, 2023
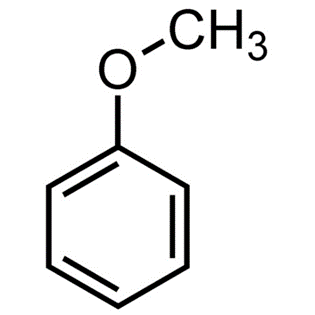
Anisole, commonly known as methoxybenzene, has the molecular formula CH3OC6H5. It does not have an odour, but it has the appearance of anise seed, and some of its derivatives are utilised in fragrances, both natural and artificial. As a scented liquid used in perfume, flavourings, insecticides, and solvents.
Chloromethyl: compounds, synthesis and safetyJun 11, 2023
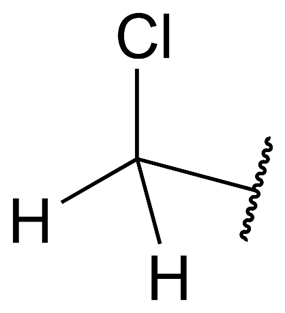
In the field of organic chemistry, a functional group known as the chloromethyl group (CH2Cl) can be found. In the formula for the methyl group (CH3), one hydrogen atom was replaced with a chlorine atom, which resulted in the creation of a new chemical entity that was subsequently given a new name.
Bromophenols: types and applicationsJun 4, 2023
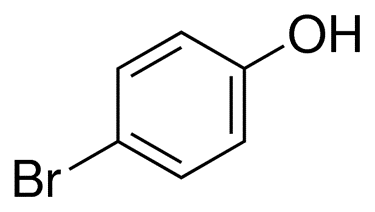
Bromophenols are the products obtained from the halogenation of phenol by electrophilic bromine. It is a powder that can range in colour from tan to orange, and it is used as an indicator of acid and base.
Bromides: History, sources, types, applications and hazards Aug 10, 2022

Bromine is located in the periodic table's halogens group, and its negatively charged form (Br) is an ion known as a bromide ion. Bromides that are colourless and have a wide range of uses, including anticonvulsants, flame retardants, and cell stains.

