21674-96-4
21674-96-4
N-[4-(2-amino-1,3-thiazol-4-yl)phenyl]acetamide
Synonyms
- 21674-96-4
- N-[4-(2-amino-1,3-thiazol-4-yl)phenyl]acetamide
- 4-(4-Acetamidophenyl)-2-aminothiazole
- N-(4-(4-AMINO-3,5-THIAZOLYL)PHENYL)ETHANAMIDE
- MFCD00207103
Molecular Formula
C11H11N3OS
Get a free no-obligation quote
We typically respond within 30 minutes during business hours!
Molecular Formula
C11H11N3OS
Iupac Name
N-[4-(2-amino-1,3-thiazol-4-yl)phenyl]acetamide
Canonical Smiles
CC(=O)NC1=CC=C(C=C1)C2=CSC(=N2)N
StdInChi
InChI=1S/C11H11N3OS/c1-7(15)13-9-4-2-8(3-5-9)10-6-16-11(12)14-10/h2-6H,1H3,(H2,12,14)(H,13,15)
StdInChiKey
VBBNSESFUHRMJU-UHFFFAOYSA-N
Molecular Weight
233.29
Exact Mass
233.06228316
Average Mass
233.290 Da
Monoisotopic Mass
233.06228316
Boiling Point
526.3±33.0 °C at 760 mmHg
Experimental Melting Point
252-256 °C
Flash Point
272.1±25.4 °C
Density
1.4±0.1 g/cm3
Vapour Pressure
0.0±1.4 mmHg at 25°C
Enthalpy of Vaporization
80.1±3.0 kJ/mol
Refractive Index
1.687
Molar Refractivity
65.8±0.3 cm3
Polar Surface Area
96.2 Ų
Polarizability
26.1±0.5 10-24cm3
Surface Tension
63.4±3.0 dyne/cm
Molar Volume
172.8±3.0 cm3
Experimental Logp
1.488
xLogP3AA
1.4
Acd LogP
1.31
Acd LogD Ph55
1.14
Acd Bcf Ph55
4.26
Acd Koc Ph55
96.05
Acd LogD Ph74
1.17
Acd Bcf Ph74
4.60
Acd Koc Ph74
103.72
Hydrogen Bond Donor Count
2
Hydrogen Bond Acceptor Count
4
Rotatable Bond Count
2
Heavy Atom Count
16
Isotope Atom Count
0
Defined Atom Stereocenter Count
0
Undefined Atom Stereocenter Count
0
Defined Bond Stereocenter Count
0
Undefined Bond Stereocenter Count
0
Covalently Bonded Unit Count
1
H Bond Acceptors
4
H Bond Donors
3
Freely Rotating Bonds
2
Rule of 5 Violations
0
Formal Charge
0
Complexity
254
Compound is Canonicalized
Yes
Related Article(s)
Aryls: Structure, nomenclature, types and applicationsAug 3, 2022
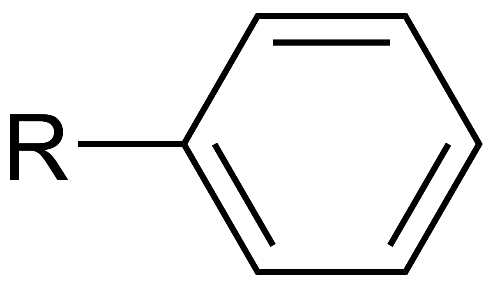
An aryl group is the name given to the functional group that is produced when an aromatic ring complex is reduced to its simplest form by removing one hydrogen atom. The aromatic ring will almost always be constituted by a hydrocarbon in most instances.
Amines: Synthesis, classification, biochemical significance, applications and hazardsJul 18, 2022
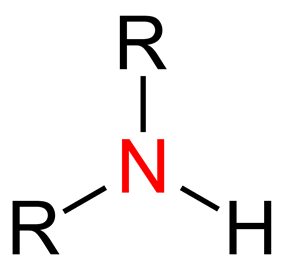
Amines are the compounds that have nitrogen atoms with a lone pair. They are either gaseous when they are at room temperature or vaporized when they are heated quickly. They have a fishy smell at low molecular weight.
Amides: Nomenclature, classification, natural sources, synthesis and applicationsJul 16, 2022
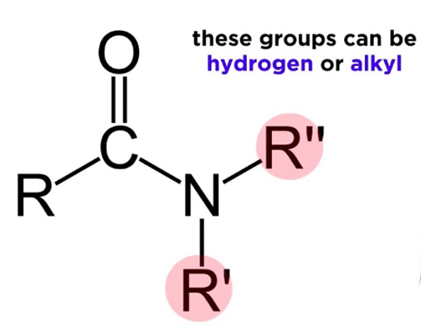
Amide is a nitrogen-containing molecule that derives from ammonia or an amine. As a functional group, amides consist of a carbonyl group joined to a nitrogen atom. Carboxylic acids and amines react to produce amides.
