63989-79-7
6-Aminocoumarin hydrochloride
Synonyms
- 6-Aminocoumarin hydrochloride
- 63989-79-7
- 6-Amino-2H-chromen-2-one hydrochloride
- MFCD00082671
- NSC613801
Molecular Formula
C9H8ClNO2
Get a free no-obligation quote
We typically respond within 30 minutes during business hours!
Molecular Formula
C9H8ClNO2
Iupac Name
6-aminochromen-2-one;hydrochloride
Canonical Smiles
C1=CC2=C(C=CC(=O)O2)C=C1N.Cl
StdInChi
InChI=1S/C9H7NO2.ClH/c10-7-2-3-8-6(5-7)1-4-9(11)12-8;/h1-5H,10H2;1H
StdInChiKey
OSIGAIXSSYAHEG-UHFFFAOYSA-N
Molecular Weight
197.62
Exact Mass
197.0243562
Average Mass
197.618 Da
Monoisotopic Mass
197.0243562
Experimental Boiling Point
396.9 °C
Experimental Melting Point
260 °C (Decomposes)
Polar Surface Area
52.3 Ų
Experimental Logp
0.831
Hydrogen Bond Donor Count
2
Hydrogen Bond Acceptor Count
3
Rotatable Bond Count
0
Heavy Atom Count
13
Isotope Atom Count
0
Defined Atom Stereocenter Count
0
Undefined Atom Stereocenter Count
0
Defined Bond Stereocenter Count
0
Undefined Bond Stereocenter Count
0
Covalently Bonded Unit Count
2
Formal Charge
0
Complexity
225
Compound is Canonicalized
Yes
Related Article(s)
Esters: Structure, synthesis and applicationsJul 22, 2022
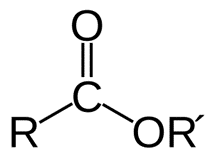
The ester group is formed when the hydrogen atom in the -COOH group of a carboxylic acid is exchanged for a hydrocarbon group. Glycerides, which are glycerol fatty acid esters, play a crucial role in living organisms.
Amines: Synthesis, classification, biochemical significance, applications and hazardsJul 18, 2022
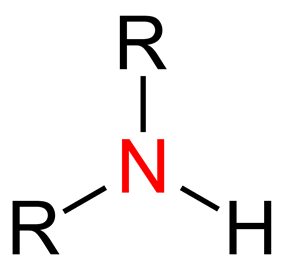
Amines are the compounds that have nitrogen atoms with a lone pair. They are either gaseous when they are at room temperature or vaporized when they are heated quickly. They have a fishy smell at low molecular weight.