6748-68-1
8-Acetyl-7-hydroxycoumarin
Synonyms
- 8-Acetyl-7-hydroxycoumarin
- 6748-68-1
- 8-acetyl-7-hydroxy-2H-chromen-2-one
- 8-acetyl-7-hydroxychromen-2-one
- 7-hydroxy-8-acetylcoumarin
Molecular Formula
C11H8O4
Get a free no-obligation quote
We typically respond within 30 minutes during business hours!
Molecular Formula
C11H8O4
Iupac Name
8-acetyl-7-hydroxychromen-2-one
Canonical Smiles
CC(=O)C1=C(C=CC2=C1OC(=O)C=C2)O
StdInChi
InChI=1S/C11H8O4/c1-6(12)10-8(13)4-2-7-3-5-9(14)15-11(7)10/h2-5,13H,1H3
StdInChiKey
XWYMACPLPPQCHC-UHFFFAOYSA-N
Molecular Weight
204.18
Exact Mass
204.04225873
Average Mass
204.179 Da
Monoisotopic Mass
204.04225873
Boiling Point
427.3±45.0 °C at 760 mmHg
Experimental Melting Point
167-171 °C
Flash Point
175.5±22.2 °C
Density
1.4±0.1 g/cm3
Vapour Pressure
0.0±1.1 mmHg at 25°C
Enthalpy of Vaporization
70.9±3.0 kJ/mol
Refractive Index
1.620
Molar Refractivity
51.7±0.3 cm3
Polar Surface Area
63.6 Ų
Polarizability
20.5±0.5 10-24cm3
Surface Tension
57.2±3.0 dyne/cm
Molar Volume
147.1±3.0 cm3
Experimental Logp
0.954
xLogP3AA
1.8
Acd LogP
1.88
Acd LogD Ph55
2.12
Acd Bcf Ph55
24.08
Acd Koc Ph55
339.10
Acd LogD Ph74
2.04
Acd Bcf Ph74
20.16
Acd Koc Ph74
283.89
Hydrogen Bond Donor Count
1
Hydrogen Bond Acceptor Count
4
Rotatable Bond Count
1
Heavy Atom Count
15
Isotope Atom Count
0
Defined Atom Stereocenter Count
0
Undefined Atom Stereocenter Count
0
Defined Bond Stereocenter Count
0
Undefined Bond Stereocenter Count
0
Covalently Bonded Unit Count
1
H Bond Acceptors
4
H Bond Donors
1
Freely Rotating Bonds
1
Rule of 5 Violations
0
Formal Charge
0
Complexity
321
Compound is Canonicalized
Yes
Related Article(s)
Alcohols: Classification, sources, commercially significant types and usesJul 31, 2022
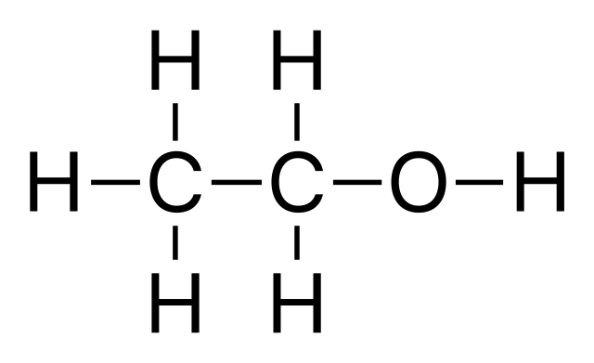
Alcohols are chemical substances that include an aliphatic carbon atom with a hydroxyl (OH) functional group attached. Because all alcohols share OH, we usually write alcohols as the general formula ROH, where R is an alkyl group.
Ketones: Nomenclature, classification, biochemical significance, applications and toxicityJul 26, 2022
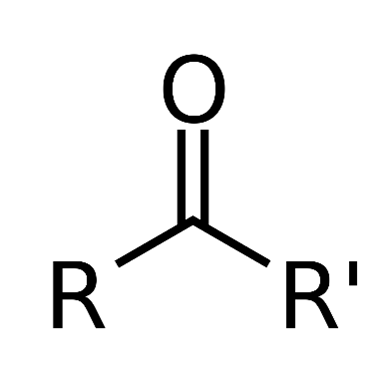
Ketones are a group of organic compounds that have a carbonyl group that is a link between oxygen and carbon. They are present in sugars and pharmaceutical chemicals, like steroid hormones.
Esters: Structure, synthesis and applicationsJul 22, 2022
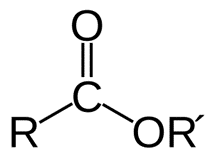
The ester group is formed when the hydrogen atom in the -COOH group of a carboxylic acid is exchanged for a hydrocarbon group. Glycerides, which are glycerol fatty acid esters, play a crucial role in living organisms.