71022-43-0
3,5-Dinitrobenzyl alcohol
- 3,5-Dinitrobenzyl alcohol
- 71022-43-0
- (3,5-Dinitrophenyl)methanol
- 3,5-DinitrobenzeneMethanol
- Benzenemethanol, 3,5-dinitro-
Get a free no-obligation quote
We typically respond within 30 minutes during business hours!
Related Article(s)
Iodobenzene: Synthesis, reactions, environmental exposure, safety and applicationsJun 25, 2023
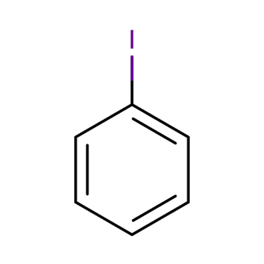
Iodobenzene is an organoiodine molecule that has one of its benzene rings switched out for an iodine atom. Iodobenzene has an empirical formula of C6H5I and a molecular weight of 204.01g/mol. In organic chemistry, it is utilised as an important intermediate in the synthesis process.
Methoxy benzene: synthesis and applicationsJun 18, 2023
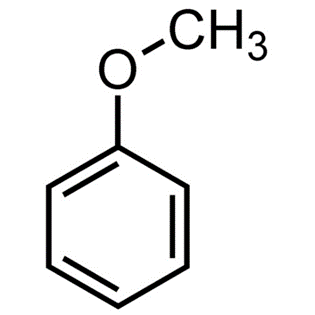
Anisole, commonly known as methoxybenzene, has the molecular formula CH3OC6H5. It does not have an odour, but it has the appearance of anise seed, and some of its derivatives are utilised in fragrances, both natural and artificial. As a scented liquid used in perfume, flavourings, insecticides, and solvents.
Benzene Compounds: Chemical structure and derived compounds Aug 7, 2022
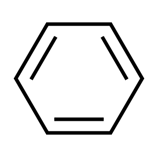
The most fundamental organic chemical is benzene, which belongs to the group of aromatic hydrocarbons. In crude oil, you may find naturally occurring benzene, as well as many other fundamental petrochemicals.
Alcohols: Classification, sources, commercially significant types and usesJul 31, 2022
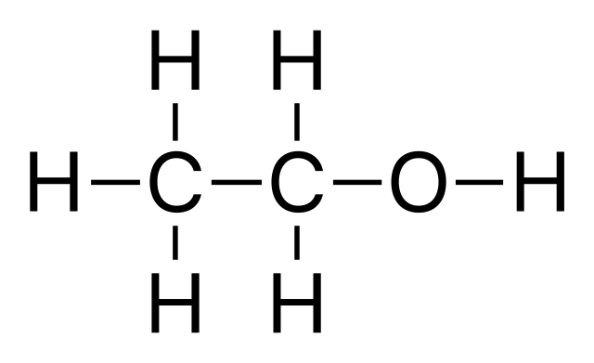
Alcohols are chemical substances that include an aliphatic carbon atom with a hydroxyl (OH) functional group attached. Because all alcohols share OH, we usually write alcohols as the general formula ROH, where R is an alkyl group.
Nitros: Synthesis, occurrence, reactions and applications Jul 28, 2022

Nitro compounds are those that have one or more nitro functional groups (NO2) in them. The nitro group is one of the most common and most often used examples of a functional group that gives a molecule explosive properties.
