7652-29-1
6-Chloro-2H-1,4-benzoxazin-3(4H)-one
- 7652-29-1
- 6-Chloro-2H-1,4-benzoxazin-3(4H)-one
- 6-chloro-2h-benzo[b][1,4]oxazin-3(4h)-one
- 6-chloro-2,4-dihydro-1,4-benzoxazin-3-one
- 6-chloro-4H-1,4-benzoxazin-3-one
Get a free no-obligation quote
We typically respond within 30 minutes during business hours!
Related Article(s)
Chloromethyl: compounds, synthesis and safetyJun 11, 2023
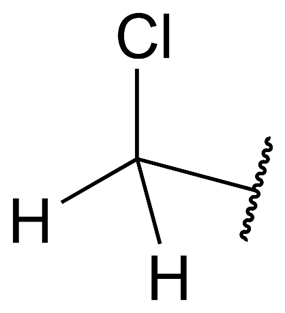
In the field of organic chemistry, a functional group known as the chloromethyl group (CH2Cl) can be found. In the formula for the methyl group (CH3), one hydrogen atom was replaced with a chlorine atom, which resulted in the creation of a new chemical entity that was subsequently given a new name.
Chlorides: Structure, primary types and applicationsAug 5, 2022

Chlorides are the negatively charged ions formed by chlorine (Cl-). To this end, chlorides are widely defined as any material containing chlorine. This category includes chlorine salts and acids such as hydrochloric acid.
Amides: Nomenclature, classification, natural sources, synthesis and applicationsJul 16, 2022
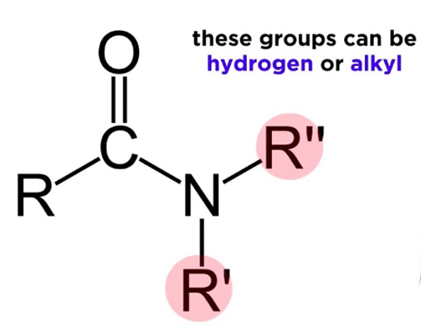
Amide is a nitrogen-containing molecule that derives from ammonia or an amine. As a functional group, amides consist of a carbonyl group joined to a nitrogen atom. Carboxylic acids and amines react to produce amides.
Chlorobenzene: Synthesis, applications and safety hazardsJun 22, 2022
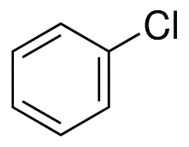
Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. It is a flammable liquid that has a clear appearance and smells like almonds to some extent.it is soluble in water but at a lower temperature.
