Alcohols Derivatives Available For Purchase
At Chempanda, we offer a range of alcoholic chemical compounds that can be shipped to anywhere across the globe. We offer over 15420 different compounds that are available in varying purities and quantities.
Our Alcohol Suppliers
We have partnered with a range of chemical manufacturers who specialize exclusively in synthesizing alcoholic compounds and their derivatives. With years of industry experience, our suppliers offer the most common alcoholic compounds, or can custom manufacture alcoholic compounds that you cannot purchase from most chemical suppliers.
With all orders, you can order the exact amount of chemical that you need - with no minimum order - in a cost and time effective manner that other suppliers cannot match.
Alcohol Physical Properties & Structure
Alcohols are a class of organic compounds that contain at least one hydroxyl group that is bound to a saturated carbon. The most common of all alcohols conform to the formula - CnH2n+1OH - with there being three main classes of alcohols; primary, secondary and tertiary.
The properties of alcohol compounds are determined by the chemical formula of the alcohol. Simple, primary alcohols - such as methanol and ethanol - are free flowing liquids and highly water soluble. Alcohols with longer carbon chains or that are highly branched tend to be physically viscous, with varying reactivity based on their chains.
Alcohol Derivatives
The number of alcohol compounds is wide ranging, with many different derivatives of alcohols available. The simplest alcohol compound - methanol - is commonly used in the production of formaldehyde which has wide ranging industrial applications.
The next simplest alcohol - ethanol - is most commonly associated with alcoholic drinks. But it is widely used in personal and home care products, as an additive or even more commonly as a fuel.
Another class of alcoholic compounds - glycols - contain two hydroxyl groups, with common compounds including ethylene glycol and propylene glycol. These compounds can be used to make a range of complex alcoholid compounds, which can be used as coatings, plasticizers or as solvents for example.
The Reactivity of Alcohols
Due to the presence of hydroxyl groups on alcohols, the electronegativity of the oxygen is greater than that between carbon and hydrogen, making the hydroxyl group the most reactive site on an alcoholic molecule.
Alcohols undergo a series of different reactions. Alcohols can be converted - via dehydration - into alkenes under the presence of heat, and can be catalyzed in the presence of acid. Alcohols will also react with alkyl halides, in a substitution reaction to form alkyl halides and water.
But alcohols will also undergo different reactions including reactions with metals, esterification and oxidation with a range of species.
Applications of Alcohols
The most common use of alcohol comes in the form of ethanol, which is widely consumed in alcoholic drinks. However, ethanol is widely used in industrial applications as a solvent, fuel or even as a material.
Other simple alcohols - methanol and propan-2-ol for example - are used as simple additives, or even in the synthesis of more complex alcohols or other materials.
Related Article(s)
Alcohols: Classification, sources, commercially significant types and usesJul 31, 2022
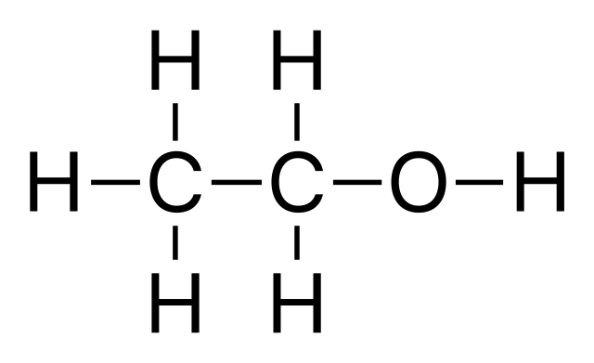
Alcohols are chemical substances that include an aliphatic carbon atom with a hydroxyl (OH) functional group attached. Because all alcohols share OH, we usually write alcohols as the general formula ROH, where R is an alkyl group.