Amide & Amide Derivatives Available For Purchase
At Chempanda, we offer a range of amide and amide derivative chemical compounds that can be shipped to anywhere across the globe. We offer over 33380 different compounds that are available in varying purities and quantities.
Our Amide Suppliers
We have partnered with a range of chemical manufacturers who specialize exclusively in synthesizing amides and their derivatives. With years of industry experience, our suppliers offer the most common amide compounds, or can custom manufacture amide compounds that you cannot purchase from most chemical suppliers.
With all orders, you can order the exact amount of chemical that you need - with no minimum order - in a cost and time effective manner that other suppliers cannot match.
Amides Physical Properties & Structure
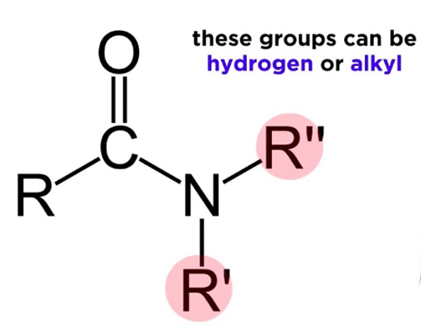
Organic amides - also known as carboxamides - are organic compounds with the general formula RC(=O)NR’R’’, with functional groups R, R’ and R’’ representing organic groups or hydrogen atoms.
Amides are derived from carboxylic acids, swapping the -COOH group with a CONH2. This forms polar amide bonds, allowing amides to readily form hydrogen bonds with water, although the solubility will depend on the molar mass of the hydrocarbon chain. Amides are typically classified as primary, secondary or tertiary amides depending on the amine subgroup present.
Amide Derivatives
Amides are primarily formed as derivatives of carboxylic acids in which the -OH part of the -COOH group is replaced by a -NH2 group. Some common amide derivatives that are used widely include carboxamides, sulfonamides and phosphoramides, which are chain amides. Although less common, cyclic amides are known as lactams.
The Reactivity of Amides
Amide compounds are susceptible to chemical reactions via the carbonyl double bond. However, their reactivity is less than other carboxylic acid derivatives due to the reduced electrophilicity caused by the lone pair on the nitrogen.
Amides can be converted into primary, secondary and tertiary amine compounds by reacting with LiAlH4. Furthermore, amides can undergo an organic reaction from a primary amide to primary amine - with one fewer carbon - in the presence of bromine and sodium hydroxide.
Applications of Amides & Derivatives
The use of amides is most commonly known for their uses in structural materials, including Kevlar and nylon.
But amides play an incredibly important role in the production of medicines and pharmaceuticals, ranging from everyday medicines to specialized surgical drugs including local and epidural anesthesia.
Related Article(s)
Amides: Nomenclature, classification, natural sources, synthesis and applicationsJul 16, 2022
Amide is a nitrogen-containing molecule that derives from ammonia or an amine. As a functional group, amides consist of a carbonyl group joined to a nitrogen atom. Carboxylic acids and amines react to produce amides.