Amine & Amine Derivatives Available For Purchase
At Chempanda, we offer a range of amine and amine derivative chemical compounds that can be shipped to anywhere across the globe. We offer over 46150 different compounds that are available in varying purities and quantities.
Our Amine Suppliers
We have partnered with a range of chemical manufacturers who specialize exclusively in synthesizing amines and their derivatives. With years of industry experience, our suppliers offer the most common amine compounds, or can custom manufacture amine compounds that you cannot purchase from most chemical suppliers.
With all orders, you can order the exact amount of chemical that you need - with no minimum order - in a cost and time effective manner that other suppliers cannot match.
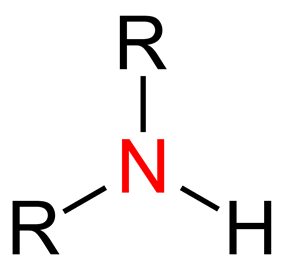
Amine Physical Properties & Structure
Amines refer to compounds (or a single functional group) that contains a basic nitrogen with a lone pair. The simplest form of an amine is ammonia NH3, but complex amine compounds can exist where one or more of the hydrogen atoms are replaced with an alkyl or aryl group meaning that amines are generally represented as R-CO-NR’R’’, where R represents functional groups.
Based on the number functional groups attached to the Nitrogen atom, will determine whether an amine classifies as a primary, secondary or tertiary amine. Additionally, it is possible to have cyclic amines; 3-membered rings known as aziridines and a 6-membered ring, piperidine.
Amine Derivatives
Amines typically derive from ammonia, but there are still many different amine derivatives that are widely used. Common amine derivatives include alkyl amines - primary amines such as amino acids and methalamines, amine salts such as cyclohexylamine and dimethylamine, and aniline derivatives.
The Reactivity of Amine
In organic chemistry, amines are widely used in the synthesis of amine derivatives, such as amino acids, amino alcohols and azo compounds. This is largely due to the wide range of reactivity from amines.
Due to the lone electron pair, amines act as Bronsted bases, readily reacting with acids to form various salts. Additionally, amines are also nucleophiles meaning that they bond to form products with electrophiles. A range of alkylation and reduction reactions can take place to form primary, secondary and tertiary amines, which can form pure products with high yields.
Applications of Amines & Derivatives
Due to the reactivity of amines, and the subsequent products that they help synthesize, amines are responsible for many wide-ranging industrial applications.
Azo compounds are commonly used as dyes and pigments, making them ideal for textiles and coatings. They are also used widely in chemical processing industries, agriculture and food.
Amines are also used widely in the health and pharmaceutical industry. Amines are used as solvents and stimulants, and are also found in common medication such as pain killers, anesthetics and even over the counter medicines.
Related Article(s)
Amines: Synthesis, classification, biochemical significance, applications and hazardsJul 18, 2022
Amines are the compounds that have nitrogen atoms with a lone pair. They are either gaseous when they are at room temperature or vaporized when they are heated quickly. They have a fishy smell at low molecular weight.