Amides: Nomenclature, classification, natural sources, synthesis and applications
As a functional group, amides consist of a carbonyl group joined to a nitrogen atom. Carboxylic acids and amines react to produce amides and it is an ionic compound with a chemical formula NH2. This compound is the conjugate base of ammonia (NH3). The chemical structure of an amide can be written as following:
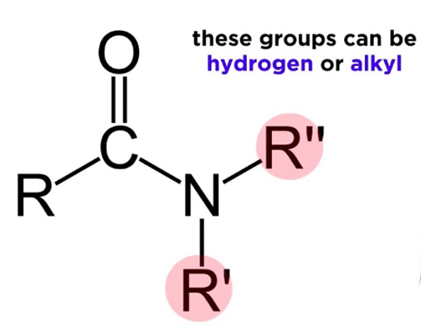
A nitrogen-containing molecule that derives from ammonia or an amine is called an amide. A neutral or slightly acidic covalent amide is formed when the hydroxyl group acid is replaced by an amino group. The most crucial kind are carboxamides, which are derivatives of carboxylic acids. Chemically, sulfonamides (RSO2NR2) are related to sulfonic acids (RSO3H).
It is possible to write out the chemical structure of an amides as follows:
Ionic, or saltlike, amides are very alkaline chemicals formed by treating ammonia, amines, and covalent amides with a reactive metal like sodium. All ammonia-based covalent amides are solids, with the exception of formamide (a liquid); those with less than five carbon atoms are soluble in water. It is possible for them to dissolve both organic and inorganic substances since they do not conduct electricity. Boiling points are high for all covalent amides, even the low molecular weight ones.
Nomenclature
The name of the amide is usually formed by adding the suffix "amide" to the original name of the parent acid. One example of an amide is acetamide, which forms when acetic acid is dissolved in water (CH3CONH2). Although ethanamide is endorsed by the IUPAC (International Union of Pure and Applied Chemistry), the recommended or related formal nomenclature is rarely used. The nitrogen substituents come first in the naming of amides generated from primary and secondary amines. When dimethylamine and acetic acid are combined, they produce the amide N, N-dimethylacetamide (CH3CONMe2, where me = CH3). Even when the longer term is used, it is commonly abbreviated to "dimethylacetamide." Since lactams are amides, they must be either secondary or tertiary amides because of their cyclic nature.
Classification
Amides are categorized into three different levels based on their names: primary, secondary, and tertiary. Nitrogen-to-carbon bonding location is used to categorize the variations. If you want to refer to a primary amide without adding 'ic acid' or 'oic acid,' you should remove the suffix.
Simply by adding a N to the name, we can observe that nitrogen has connected to the alkyl group, transforming the compound into an amide. Alkyl groups are segments of a hydrocarbon chain that only contain hydrogen and carbon atoms.
The amide group includes compounds such as carboxyl amides, sulfonamides, and phosphoramides. Nylon is a kind of polyamide. Amides include the anti-inflammatory medicines paracetamol, penicillin, and LCD.
The chemical structures of primary, secondary and tertiary amides can be written as following:

Natural Sources
Amides are present in very little amount in nature. In contrast to polyamides, which are found in abundance as the proteins of living systems, simple covalent amides cannot be produced sustainably from any natural source. Simple amides are often generated by reaction of acids or halides of acid and amines. Water is added to nitriles to create these compounds.
Synthesis
- The amide is produced via a reaction between the ammonium salt of the carboxylic acid and heat. The solid NH4CO3 reacts with acid in surplus amount to produce the NH4 salt. The reaction of an excess of ethanoic acid with ammonium carbonate produces ammonium ethanoate.
- At the end of the process, the ammonium salt dehydrates when heated, yielding ethanamide.
- In order to prevent the ammonium salt from dissociating before dehydration, an excess of ethanoic acid must be present. Dissociation would cause ammonia to be lost from the reaction mixture at these conditions. Absolutely no recombination could take place. Restoration of the divide is possible. This is because the excess ethanoic acid, which acts as a buffer, has shifted the equilibrium.
- Amides are often made from acid chlorides, acid azides, acid anhydrides, and acid esters. Making them directly from amines and carboxylic acids is difficult without first subjecting the mixture to high temperatures or coupling it to a second procedure that "activates" the acid. Since phenols are stronger acids than alcohols, their esters have a greater propensity to react with amines.
Uses
The following are some of the ways in which amides are useful:
- There are a variety of reasons why plants produce amides. Amides are incorporated in several different classes of medications.
- The amide family includes numerous common medications, including penicillin, LSD, and paracetamol. Furthermore, plant N-alkylamides participate in a wide range of biological processes.
- Solvents like dimethylformamide and acetamide (also known as ethanamide) are amides, as are sulfa drugs and nylon.
- Crystalline urea, also called carbamide, is a byproduct of protein metabolism in mammals. To produce urea-formaldehyde resins, a kind of polymer used in the plastics industry, ammonia and carbon dioxide are combined in large amounts.