Methylphenol: Common isomers, structure, synthesis, applications and natural sources
Cresols is an aromatic chemical family including the compounds like hydroxytoluenes and cresylic acids and the methylphenols. Methylphenols are the most well-known of this family. The chemical structure of methylphenols is represented by the formula CH3C6H4(OH), and its molar mass is 108.140 gmol1. They are a derivative of phenol with a methyl group in its structure. Methylphenols, which are also known as phenolics, are found often in nature and may also be synthesized artificially in a laboratory. Because their melting temperatures are so near to the temperature of the surrounding environment, these compounds may exist in both the solid and liquid states.
Structure
Methylphenols are a kind of phenol derivative in which a methyl group has been inserted into the molecule of the original phenol.
The majority of methylphenols exist in one of three possible isomeric forms:
- 2-methylphenols (ortho-cresol)
- 3-methylphenols (meta-cresol)
- 4-methylphenols (para-cresol)
Their chemical structures can be written as:
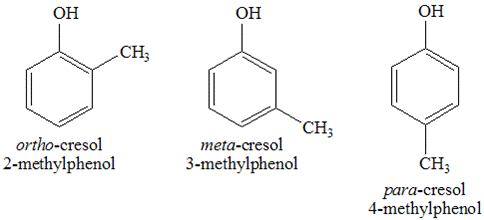
A chemical known as tricresol has been given this name because it contains all three of these isomers.
Synthesis
Coal tar is the usual ingredient in the production of all of them. After performing an alkylation on the phenol ring, some ones of them can also be produced by hydrolyzing sulphonates with methanol.
2-Methylphenol (ortho-cresol)
It is an organic compound with the molecular formula CH3C6H4 with an addition of (-OH) group. The odorless and colorless solid is an essential component in a wide variety of production procedures. It is also represented by the following names:
- ortho-cresol
- 2-Hydroxytoluene
- acid o-cresylic
Natural sources
Castor glands of beavers and the cedar that beavers eat both contain 2-methylphenols (ortho-cresol), a substance that occurs naturally in the environment. In addition, the person's usage of tobacco products, specifically cigarettes.
Synthesis
During the manufacturing process of coal-based energy, 2-methylphenol, along with a large number of other aromatic organic compounds, may be created from tar and coke. 2-Methylphenol is another compound that derives from waste products of the petroleum industry. In addition to this, a large number of other phenols and methylphenols are produced. The addition of a methyl group to phenols in the presence of methanol results in the production of approximately three quarters of these compounds; the remaining chemicals are those that occur naturally.
C6H5OH + CH3OH = CH3C6H4OH + H2O.
Uses
Methylphenols are used by businesses in a variety of industries, including agriculture, medicine, the food industry, and chemistry.
When 2-Methylphenol is nitrified, a powerful herbicide called dinitrocresol is produced. However, when the chemical is chlorinated, a useful broad-leaf herbicide called 2-methyl-4-chlorophenoxyacetic acid is produced (MCPA).
Carboxylation of 2-methylphenol, a chemical process, results in the production of o-cresotinic acid, which has use in the pharmaceutical industry. By performing an alkylation of 2-Methylphenol with propene, this chemical may be utilised to generate a synthetic version of oregano essence. This molecule is the starting point for the production of the muscle relaxant mephenesin, which is utilised in human medicine.
3-methylphenols (meta-cresol)
The chemical formula for 3-methylphenol is CH3C6H4, and it is classified as an organic phenolic compound (OH). This isomer is utilised in the synthesis of a number of different compounds that have extensive application as a building block. It has a molar mass of 108.14 gmol-1. 3-methylphenol, also known as m-Cresol.
Meta-cresol, in addition to other isomers, is naturally present in the tar and coke that are generated from coal. The number assigned to it by CAS is 108-39-4. A solution consisting of methallyl chloride and acetylene can have a molecule of carbon monoxide introduced into it with the help of nickel carbonyl. It is also known as 3-Hydroxytoluene, m-Cresylic acid, and 1-Hydroxy-3-methylbenzene, among its many other names. 3-Methylphenol is a compound that has three methyl groups.
Uses
- m-Cresol has several uses since it is used as a component in the production of a wide variety of other chemicals.
- This chemical is a component in the production of many insecticides, such as fenitrothion and fenthion.
- When 3-Methylphenol is methylated, the product that is produced is called 2, 3, 6-trimethylphenol (synthetic vitamin E).
- When 3-Methylphenol is heated, a number of different antiseptics are produced, and amylmetacresol is one of them.
- These are used for the storage of insulin.
- They are also used for production of flavourings using synthetic ingredients
- This material can be utilised to make compounds that can be used in the secondary doping process, and it can do so through the production of other compounds.
4-methylphenols (para-cresol)
4-Methylphenol is a colourless solid that may be found in abundance in coal tar, coke, and petroleum residuals. It is also known as p-cresol, 4-Hydroxytoluene, 1-Hydroxy-4-methylbenzene, and 4-cerol. This isomer is similar to the other isomers of methylphenols.
Synthesis
It can be synthesized by sulfonating toluene in order to get the equivalent sulphonate salt. Although it is a phenol derivative, it can also be synthesized in this way.
CH3C6H5 + H2SO4 CH3C6H4SO3H + H2O
Hydrolysis results in the production of cresol and sodium salt.
The result of the reaction that takes place between two sodium hydroxide (NaOH) and one methyl sulphate (CH3C6H4SO3H) is methyl sulphate (CH3C6H4OH), as well as sodium sulphate (Na2SO4).
Uses
The production of several different types of antioxidants, such as butylated hydroxytoluene, requires the usage of 4-methylphenol (BHT). Additionally, derivatives of the diphenol antioxidant family may be produced using this molecule as a starting material. The antioxidants that may be produced with the help of this chemical are in great demand because of their low toxicity and absence of the ability to stain.
Natural Sources
This isomer is produced when proteins in the human digestive system undergo the process of fermentation, which results in its subsequent elimination from the body in the form of urine, faeces, and sweat. P-cresol, which may be detected in cigarette smoke, is also a carcinogen. In addition to that, it has been discovered in the temporal excretions of male elephants as well as in the smell that pigs give off.