Synonyms
- N-(4-Oxocyclohexyl)acetamide
- 27514-08-5
- 4-Acetamidocyclohexanone
- 4-acetamido-cyclohexanone
- 4-(Acetylamino)cyclohexanone
Molecular Formula
C8H13NO2
Get a free no-obligation quote
We typically respond within 30 minutes during business hours!
Molecular Formula
C8H13NO2
Iupac Name
N-(4-oxocyclohexyl)acetamide
Canonical Smiles
CC(=O)NC1CCC(=O)CC1
StdInChi
InChI=1S/C8H13NO2/c1-6(10)9-7-2-4-8(11)5-3-7/h7H,2-5H2,1H3,(H,9,10)
StdInChiKey
WZEMYWNHKFIVKE-UHFFFAOYSA-N
Appearance
Off-white Powder
Molecular Weight
155.19
Exact Mass
155.094628657
Average Mass
155.194 Da
Monoisotopic Mass
155.094628657
Boiling Point
359.1±31.0 °C at 760 mmHg
Experimental Boiling Point
359.1 °C
Experimental Melting Point
137 °C
Flash Point
168.9±25.0 °C
Density
1.1±0.1 g/cm3
Vapour Pressure
0.0±0.8 mmHg at 25°C
Enthalpy of Vaporization
60.5±3.0 kJ/mol
Refractive Index
1.476
Molar Refractivity
40.9±0.4 cm3
Polar Surface Area
46.2 Ų
Polarizability
16.2±0.5 10-24cm3
Surface Tension
36.9±5.0 dyne/cm
Molar Volume
145.0±5.0 cm3
Experimental Logp
-0.112
xLogP3AA
-0.2
Acd LogP
-1.02
Acd LogD Ph55
-0.26
Acd Bcf Ph55
1.00
Acd Koc Ph55
17.29
Acd LogD Ph74
-0.26
Acd Bcf Ph74
1.00
Acd Koc Ph74
17.29
Hydrogen Bond Donor Count
1
Hydrogen Bond Acceptor Count
2
Rotatable Bond Count
1
Heavy Atom Count
11
Isotope Atom Count
0
Defined Atom Stereocenter Count
0
Undefined Atom Stereocenter Count
0
Defined Bond Stereocenter Count
0
Undefined Bond Stereocenter Count
0
Covalently Bonded Unit Count
1
H Bond Acceptors
3
H Bond Donors
1
Freely Rotating Bonds
1
Rule of 5 Violations
0
Formal Charge
0
Complexity
167
Compound is Canonicalized
Yes
Related Article(s)
Ketones: Nomenclature, classification, biochemical significance, applications and toxicityJul 26, 2022
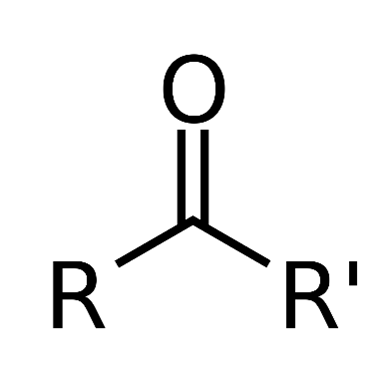
Ketones are a group of organic compounds that have a carbonyl group that is a link between oxygen and carbon. They are present in sugars and pharmaceutical chemicals, like steroid hormones.
Amines: Synthesis, classification, biochemical significance, applications and hazardsJul 18, 2022
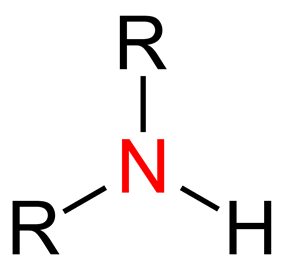
Amines are the compounds that have nitrogen atoms with a lone pair. They are either gaseous when they are at room temperature or vaporized when they are heated quickly. They have a fishy smell at low molecular weight.
Amides: Nomenclature, classification, natural sources, synthesis and applicationsJul 16, 2022
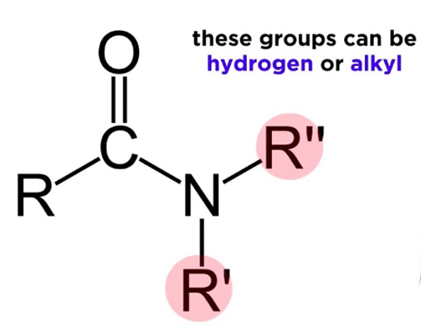
Amide is a nitrogen-containing molecule that derives from ammonia or an amine. As a functional group, amides consist of a carbonyl group joined to a nitrogen atom. Carboxylic acids and amines react to produce amides.
